Acids, Bases and Salts Unit Plan
Period/Topic Worksheets Quiz
1. Properties of Acids, Bases & Salts WS 1
2. Arrhenius, Bronsted Acids, Ka and Strength. WS 2 1
3. Arrhenius, Bronsted Bases, Kb and Strength WS 3
4. Acid & Base Reactions. Amphiprotic. Acid Chart. WS 4 2
5. Leveling effect, Anhydrides and Relationships. WS 5
6. Hydrolysis of Salts. Quiz. WS 6 3
7. Acid, Base & Salt Reactions. Hydrolysis. WS 7
8. Yamada’s Indicator Lab. Hydrolysis. WS 8 4
9. Ionization of Water, [H+] & [OH-], pH scale. WS 9
10. pH Calculations for Weak Acids. WS 10 5
11. Ka from pH for Weak Acids. WS 11
12. Indicators Lab.
13. Kbs from Kas for Weak Bases. WS 12 6
14. pH for Weak Bases pH [H+] [OH-] Relationships. WS 13
15. Amphiprotic Ions- Kas and Kbs. WS 14 7
16. Titration Lab. Primary Standards. Acids Midterm Practice Test
17. Titration Lab
18. Buffers & Indicators WS 15 8
19. Titration Curves. WS 16 9/10
20. Review # 1 Web Site Review Practice Test 1
21. Review # 2 Practice Test 2
22. Test
Worksheet # 1 Properties of Acids and Bases
1. Add 1 drop of each solution to 1 drop of the acid-base indicator in a spot plate. Record the colour in the data table below. Describe each solution as an acid or base in the space provided. Write the acid colour and base colour in the table below.
Indicator Phenolphthalein Litmus BromothymolBlue Acid/Base
Solution
HCl
NaOH
Vinegar
Ammonia
(NH3)
Lemon Juice
Seven-up
Baking Soda
(NaHCO3)
Indicator Acid Colour Base Colour
Phenolphthalein
Litmus
Bromothymol Blue
Wash and dry your spot plate before going on to step 2.
2. Wear safety goggles for this experiment. Pour approximately 50 mL of 1 M HCl into a fleaker. Add one level spoonful of Ca and cover with a plastic funnel. After 1 minute and not before light the top of the funnel using a match. Write the equation for the reaction below.
Wash and dry your fleaker before going on to step 3.
3. Taste a lemon and describe the taste in one word
4. Taste some baking soda and describe the taste in one word.
6. Test two
drops of HCl for conductivity in a spot plate. Result:
Write an equation that accounts for the conductivity of HCl.
7. Test two
drops of NaOH for conductivity in a spot plate. Result:
Write an equation that accounts for the conductivity of NaOH (dissociation).
Clean, dry and put away the spot plate
8. List five properties of acids that are in your textbook.
9. List five properties of bases that are in your textbook.
10. Make some notes on the commercial acids: HCl and H2SO4 .
HCl
H2SO4
11. Make some notes on the commercial base NaOH.
12. Describe the difference between a concentrated and dilute acid (hint: concentration refers to the molarity). Describe their relative conductivities.
13. Describe the difference between a strong and weak acid. Use two examples and write equations to support your answer. Describe their relative conductivities.
14. Describe a situation where a strong acid would have the same conductivity as a weak acid (hint: think about concentration).
Worksheet # 2 Conjugate Acid-Base Pairs
Complete each acid reaction. Label each reactant and product as an acid or base. The first on is done for you.
1. HCN + H2O ⇄ H3O+ + CN-
Acid Base Acid Base
2. H3C6O7 + H2O ⇄
3. H3PO4 + H2O ⇄
4. HF + H2O ⇄
5. H2CO3 + H2O ⇄
6. NH4+ + H2O ⇄
7. CH3COOH + H2O ⇄
8. HCl + H2O ⇄
9. HNO3 + H2O ⇄
Write the equilibrium expression (Ka) for the first seven above reactions. The first one is done for you.
10. Ka = [H3O+][CN-] 14. Ka =
[HCN]
11. Ka = 15. Ka =
12. Ka = 16. Ka =
13. Ka =
17. Which acids are strong?
18. What does the term strong acid mean?
19. Why is it impossible to write an equilibrium expression for a strong acid?
20. Which acids are weak?
23. What does the term weak acid mean?
24. Explain the difference between a strong and weak acid in terms of electrical conductivity.
Acid Conjugate Base Base Conjugate Acid
14. HNO2 15. HCOO-
16. HSO3-
17. IO3-
18. H2O2 19. NH3
20. HS- 21. CH3COO-
22. H2O 23. H2O
Define:
22. Bronsted acid-
23. Bronsted base-
24. Arrhenius acid-
25. Arrhenius base-
26. List the six strong acids.
27. Rank the acids in order of decreasing
strength. HCl H2S H3PO4 H2CO3 HF
HSO4
28. What would
you rather drink vinegar or hydrochloric acid? Explain.
Making a Universal Indicator Lab Activity
Mix the following indicators in a 50 mL beaker. Stir with an eyedropper.
Yamada’s Universal Indicator
5 drops thymol blue
6 drops methyl orange
5 drops phenolphthalein
10 drops bromothymol blue
20 drops of water
Part 1. In a spot plate add two drops of each buffer solution to a cell. Add one drop of Yamada’s indicator to each. Record each colour on another lab sheet by colouring the cell the same colour. Make sure you are accurate because you will use this information for future labs and projects.
<---------- Acid Strength Increases ------ Neutral ----Base
Strength Increases ------->
|
pH = 1 |
pH = 3 |
pH = 5 |
pH = 7 |
pH = 9 |
pH =11 |
pH = 13 |
|
|
|
|
|
|
|
|
Part 2. Test a drop of HCl, CH3COOH, NaOH, NH3, NaHCO3, H2CO3 and NaCl solution for conductivity. Test with your Universal Indicator. Record the pH of each. Test with your Universal Indicator. Explain your results with what you know about acids and bases. Classify each as a strong or weak acid or base or neutral, acidic, or basic salt. Write an equation for each to show how they ionize in water using the Bronsted (Chemistry 12) definition of an acid.
Wash and dry your chem plate
Wash and return your eyedropper.
Wash and return your beaker.
Wash your hands.
Results
Compound Conductivity pH Classification
HCl
CH3COOH
NaOH
NH3
NaHCO3
H2CO3
NaCl
Worksheet # 3 Conjugate Acid-Base Pairs
Complete each reaction. Label each reactant and product as an acid or base.
1. HCN + H2O ⇄ H3O+ +
CN-
Acid Base Acid Base
2. HCl + H2O ⇄
3. HF + H2O ⇄
4. F- + H2O ⇄
5. HSO4- + H2O ⇄
(acid)
6. NH4+ + H2O ⇄
7. HPO42- + H2O ⇄
(base)
Acid Conjugate Base Base
Conjugate Acid
8. HCO3- CO32- 9. CH3COO- CH3COOH
10. HPO42- 11. IO3-
12. H2O 13. NH2-
14. HS- 15. C2H5SO73-
16. Circle the strong bases.
Fe(OH)3 NaOH CsOH KOH
Zn(OH)2 Sr(OH)2 Ba(OH)2 Ca(OH)2
17. Rank the following acids from strongest to weakest.
H2S CH3COOH H2PO4- HI HCl HF
18. Rank the following bases from the strongest to weakest.
H2O F- NH3 SO32- HSO3- NaOH
19. i) Write the reaction of H3BO3 with water (remove one H+ only because it is a weak acid).
ii) Write the Ka expression for the above.
iii) What is the ionization constant for the acid (use your table). Ka =
20. List six strong acids.
21. List six strong bases.
22. List six weak acids in order of decreasing strength (use your acid/base table).
23. List six weak bases in order of decreasing strength (use your acid/base table).
Worksheet # 4 Using Acid Strength Tables
Acid-base reactions can be considered to be a competition for protons. A stronger acid can cause a weaker acid to act like a base. Label the acids and bases. Complete the reaction. State if the reactants or products are favoured.
1. HSO4- + HPO42- ⇄
2. HCN + H2O ⇄
3. HCO3- + H2S ⇄
4. HPO42- + NH4+ ⇄
5. NH3 + H2O ⇄
6. H2PO41- + NH3 ⇄
7. HCO3- + HF ⇄
8. Complete each equation and indicate if reactants or products are favoured. Label each acid or base.
HSO4- + HCO3- ⇄
H2PO4- + HC03- ⇄
HS03- + HPO42- ⇄
NH3 + HC2O4- ⇄
9. Explain why HF(aq) is
a better conductor than HCN(aq).
10. Which is a stronger acid in water, HCl or HI? Explain!
11. State the important ion produced by an acid and a base.
12. Which is the stronger base? Which produces the least OH-? F- or CO3-2
13. Define a Bronsted/Lowry acid and base.
14. Define an Arrhenius acid and base.
15. Complete each reaction and write the equilibrium expression.
HF + H2O ⇄ Ka=
F- + H2O ⇄ Kb=
16. H2SO4 + NaOH →
17. Define conjugate pairs.
18. Give
conjugate acids for: HS-, NH3,
HPO4-2, OH-, H2O, NH3,
CO3-2
19. Give conjugate bases for: NH4+, HF,
H2PO4-, H3O+, OH-,
HCO3-, H2O
Worksheet # 5 Acid and Basic Anhydrides
1. What is the strongest acid that can exist in water? Write an equation to show how a stronger acid would be reduced in strength by the leveling effect of water.
2. What is the strongest base that can exist in water? Write an equation to show how a stronger base would be reduced in strength by the leveling effect of water.
3. List three strong acids and three strong bases.
4. Rank the acids in decreasing strength:
HClO4 Ka
is very large HClO3 Ka=1.2x10-2
HClO2 Ka=8.0x10-5 HClO Ka=4.4x10-8
5. For an oxy acid what is the relationship between the number of O’s and acid strength? (Compare H2S04 and H2S03)
6. Which acid is stronger? HI03 or HIO2
7. Which produces more H30+? H2CO3 or HS04-
8. Which produces more OH-? F- or HC03-
9. Which conducts better NH3 or NaOH (both .1M)? Why?
10. Which
conducts better HF or HCN (both .1M)? Why?
11. Compare and contrast a strong and weak
acid in terms of degree of ionization, size of ka, conductivity, and
concentration of H+.
Classify each formula as an acid anhydride, basic anhydride, strong acid, weak acid, strong, or weak base. For each formula write an equation to show how it reacts with water. For anhydrides write two equations.
Formula Classification Reaction
12. Na2O
13. CaO
14. SO3
15. CO2
16. SO2
17. HCl
18. NH3
19. NaOH
20. HF
21. H3PO4
Worksheet # 6 Hydrolysis of Salts and Reactions of Acids and Bases
Describe each as an acid, base, neutral salt, acidic salt, or basic salt. For each salt write a parent acid-base formation equation, dissociation equation, and hydrolysis equation (only for acidic and basic salts). For acids and bases write an equation to show how each reacts with water.
1. NH3
2. KCl
3. HNO3
4. NaHCO3
5. RbOH
6. AlCl3
7. H2C2O4
8. NaC6H5O
9. Co(NO3)3
10. Na2CO3
Worksheet # 7 Hydrolysis of Salts and Reactions of Acids and Bases
Describe each as an acid, base, neutral salt, acidic salt, or basic salt. For each salt write a dissociation equation and hydrolysis equation (only for acidic and basic salts). For acids and bases write an equation to show how each reacts with water.
1. NH3
2. NaCl
3. HCl
4. NaCN
5. NaOH
6. FeCl3
7. HF
8. LiHCO3
9. Fe(NO3)3
10. MgCO3
11. H2S
12. HF
13. CaI2
14. Mg(OH)2
15. Ba(OH)2
16. Describe why Tums (CaCO3) neutralizes stomach acid.
17. Describe why Mg(OH)2 is used in Milk of Magnesia as an antacid instead of NaOH.
Worksheet # 8 Yamada’s Indicator Activity
Acid, Base and Salt Lab
Purpose:
1) To use Yamada’s Indicator to determine the pH of various acids, bases and salts.
2) To classify compounds as strong acids, weak acids, strong bases, weak bases, neutral salts, acid anhydrides, and basic anhydrides.
3) To write reactions for each compound to show how each ionizes, hydrolyzes or reacts with water.
Procedure:
1) To a cell in a spot plate add one drop of solution or a very tiny amount of solid. Write the formula of the compound in the data table.
2) Add two drops of Yamada’s Indicator. Record the pH of the compound.
3) Classify the compound as a strong acid, weak acid, strong base, weak base, neutral salt, acid anhydride, or basic anhydride. Use the formula of the compound as well as the pH.
4) Write an equation to show the reaction of anhydrides with water, the hydrolysis of salts, or the ionization of acids or bases.
Data
1. Formula of
compound
pH
Classification
Reaction or
reactions
2. Formula of
compound
pH
Classification
Reaction or
reactions
3. Formula of
compound
pH
Classification
Reaction or reactions
4. Formula of
compound
pH
Classification
Reaction or
reactions
5. Formula of
compound
pH
Classification
Reaction or
reactions
6. Formula of
compound
pH
Classification
Reaction or
reactions
7. Formula of
compound
pH
Classification
Reaction or
reactions
8. Formula of
compound
pH
Classification
Reaction or
reactions
9. Formula of
compound
pH
Classification
Reaction or
reactions
10. Formula of
compound
pH
Classification
Reaction or
reactions
11. Formula of
compound
pH
Classification
Reaction or
reactions
12. Formula of
compound
pH
Classification
Reaction or
reactions
13. Formula of
compound
pH
Classification
Reaction or
reactions
14. Formula of
compound
pH
Classification
Reaction or
reactions
15. Formula of
compound
pH
Classification
Reaction or
reactions
16. Formula of
compound
pH
Classification
Reaction or reactions
17. Formula of
compound
pH
Classification
Reaction or
reactions
Worksheet # 9 pH and pOH Calculations
Complete the chart:
|
|
[H+] |
[OH-] |
pH |
pOH |
Acid/base/neutral |
|
1. |
7.00 x 10-3 M |
|
|
|
|
|
2. |
|
8.75 x 10-2 M |
|
|
|
|
3. |
|
|
7.33 |
|
|
|
4. |
|
|
|
4.00 |
|
|
5. |
|
|
|
|
Neutral (2 sig figs) |
|
6. |
|
|
|
10.7 |
|
|
7. |
|
|
2.553 |
|
|
|
8. |
5.0 x 10-10 M |
|
|
|
|
|
9. |
|
4.7 x 10-10 M |
|
|
|
10. Calculate the [H+], [OH-], pH and pOH for a 0.20 M Ba(OH)2 solution.
11. Calculate the [H+], [OH-], pH and pOH for a 0.030 M HCl solution.
12. Calculate the [H+], [OH-], pH and pOH for a 0.20 M NaOH solution.
13. 300.0 mL of 0.20 M HCl is added to 500.0 mL of water, calculate the pH of the solution.
14. 200.0 mL of 0.020 M HCl is diluted to a final volume of 500.0 mL with water, calculate the pH.
15. 150.0 mL of 0.40 M Ba(OH)2 is placed in a 500.0 mL volumetric flask and filled to the mark with water, calculate the pH of the solution.
16. 250.0 mL of 0.20M Sr(OH)2 is diluted by adding 350.0 mL of water, calculate the pH of the solution.
17. Calculate the pH of a 0.40 solution of Ba(OH)2 when 25.0 mL is added to 25.0 mL of water.
Worksheet # 10 pH Calculations for Weak Acids
1. Calculate the [H+], [OH-], pH, and pOH for 0.20 M HCN.
[H+] = [OH-] = pH = pOH =
2. Calculate the [H+], [OH-], pH, and pOH for 2.20 M HF.
[H+] = [OH-] = pH = pOH =
3. Calculate the [H+], [OH-], pH, and pOH for 0.805 M CH3COOH.
[H+] = [OH-] = pH = pOH =
4. Calculate the [H+], [OH-], pH, and pOH for 1.65 M H3BO3.
[H+] = [OH-] = pH = pOH =
5. Calculate the pH of a saturated solution of Mg(OH)2.
6. Calculate the pH of a 0.200 M weak diprotic acid with a Ka = 1.8 x 10-6.
7. 350.0 mL of 0.20M Sr(OH)2 is diluted by adding 450.0 mL of water, calculate the pH of the solution.
Worksheet # 11 pH Calculations for Weak Acids
1. The pH of 0.20 M HCN is 5.00. Calculate the Ka for HCN. Compare your calculated value with that in the table.
2. The pH of 2.20 M HF is 1.56. Calculate the Ka for HF. Compare your calculated value with that in the table.
3. The pH of 0.805 M CH3COOH is 2.42. Calculate the Ka for CH3COOH. Compare your calculated value with that in the table.
4. The pH of 1.65 M H3BO3 is 4.46. Calculate the Ka for H3BO3. Compare your calculated value with that in the table.
5. The pH of a 0.10 M diprotic acid is 3.683, calculate the Ka and identify the acid.
6. The pH of 0.20 M NH3 is 11.227; calculate the Kb of the Base.
7. The pH of 0.40 M NaCN is 11.456; calculate the Kb for the basic salt. Start by writing an equation and an ICE chart.
8. The pH of a 0.10 M triprotic acid is 5.068, calculate the Ka and identify the acid.
9. How many grams of CH3COOH are dissolved in 2.00 L of a solution with pH = 2.45?
Use questions 1 to 4 from last assignment to mark questions 1 to 4.
Worksheet # 12 Kb For Weak Bases
Determine the Kb for each weak base. Write the ionization reaction for each. Remember that Kw = Ka • Kb (the acid and base must be conjugates). Find the base on the right side of the acid table and use the Ka values that correspond. Be careful with amphiprotic anions! The first one is done for you.
1. NaNO2 (the basic ion is NO2-)
Kb(NO2-) = Kw = 1.0 x 10-14 = 2.2 x 10-11
Ka(HNO2) 4.6 x 10-4
2. KCH3COO (the basic ion is CH3COO-)
3. NaHCO3
4. NH3
5. NaCN
6. Li2HPO4
7. KH2PO4
8. K2CO3
9. Calculate the [H+], [OH-], pH, and pOH for 0.20 M H2CO3.
[H+]
= [OH-] = pH = pOH =
10. The pH of 0.20 M H2CO3 is 3.53. Calculate the Ka for H2CO3. Compare your calculated value with that in the table.
11. Calculate the [H+], [OH-], pH, and pOH for 0.10 M CH3COOH.
[H+] = [OH-] = pH = pOH =
12. The pH of 0.10 M CH3COOH is 2.87. Calculate the Ka for CH3COOH. Compare your calculated value with that in the table.
13. 200.0 mL of 0.120 M H2SO4 reacts with 400.0 mL of 0.140 M NaOH. Calculate the pH of the resulting solution.
Worksheet # 13 Acid and Base pH Calculations
For each weak bases calculate the [OH-], [H+], pOH and pH. Remember that you need to calculate Kb first.
1. 0.20 M CN-
2. 0.010 M NaHS (the basic ion is HS-)
3. 0.067 M KCH3COO
4. 0.40 M KHCO3
5. 0.60 M NH3
6. If the pH of a 0.10 M weak acid H2X is 3.683, calculate the Ka for the acid and identify the acid using your acid chart.
7. Calculate the [H+], [OH-], pH, and pOH for 0.80 M H3BO3.
[H+]
= [OH-] = pH = pOH =
8. Calculate the [H+], [OH-], pH, and pOH for 0.25 M H2CO3.
[H+] = [OH-] = pH = pOH =
9. The pH of 1.65 M H3BO3 is 4.46. Calculate the Ka for H3BO3. Compare your calculated value with that in the table.
10. The pH of 0.65 M NaX is 12.46. Calculate the Kb for NaX.
11. Consider the following reaction: 2HCl + Ba(OH)2 → BaCl2 + 2H2O
When 3.16g samples of Ba(OH)2 were titrated to the equivalence point with an HCl solution, the following data was recorded.
Trial Volume of HCl added
#1 37.80 mL
#2 35.49 mL
#3 35.51 mL Calculate the original [HCl]
12. Calculate the volume of 0.200M H2SO4 required to neutralize 25.0 ml of 0.100M NaOH.
13. 25.0 mL of .200 M HCl is mixed with 50.0 mL 0.100 M NaOH, calculate the pH of he resulting solution.
14. 10.0 mL of 0.200 M H2SO4 is mixed with 25.0 mL 0.200 M NaOH, calculate the pH of the resulting solution.
15. 125.0 mL of .200 M HCl is mixed with 350.0 mL 0.100 M NaOH, calculate the pH of the resulting solution.
16. Define standard solution and describe two ways to standardize a solution.
17. What is the [H3O+] in a solution formed by adding 60.0 mL of water to 40.0 mL of 0.040 M KOH solution?
Worksheet # 14 Amphiprotic
Ions and Review
1. List the properties of acids/bases.
2. Define the following:
Arhenius strong acid
Arhenius weak base
Bronsted strong acid
Bronsted weak base
Conjugate pair
Amphiprotic
Standard solution.
3. Show by calculation if the following amphiprotic ions are acids or bases:
HCO3-
H2PO4-
HPO42-
4. What is the strongest base in water? What is the strongest acid in water? Write equations to explain your answer.
5. Match each equation:
Acid/base complete HCl + NaOH → NaCl + HOH
Acid/base net ionic F- + HOH →
HF + OH-
Solubility product H+ + OH- → HOH
Hydrolysis AgCl(s) → Ag+ + Cl-
Acid/Base formula H20
→ H+ + OH-
Ionization of water H+ + Cl- + Na+ + OH- → Na++ Cl- + H2O
6. HCl and HF. Describe each acid as:
a) strong or weak
b) high or low ionization
c) large or small Ka
d) good or poor conductor
e) strong or weak electrolyte
7. Out of 0.2 M HCl and 1.0 M HF, which is the most concentrated?
Which is the strongest acid?
8. Label the scale as strong/weak acid and strong/weak base.
|________________________|_________________________|__
pH 0 7 14
9. Which ions are amphiprotic?
HPO42- HCl F- HS- H2S H2O
10. Write the net ionic equation between any strong acid and strong base.
11. Write the ionization equation for water.
12. Write the Kw expression.
13. H2SO3 + HS- ⇄ H2S + HSO3-
a) Are the reactants or products favoured?
b) Is the Keq large, small or about 1?
Determine the pH Write equations for each first!
14. .20M HCl pH=?
15. 0.20M Ba(OH)2 pH=?
16. 0.20M H2CO3 pH=?
17. 0.40M KHCO3 pH=?
18. The pH increases by 2 units. How does [H+] change?
19. The pH decreases by 1 unit. How does [H+] change?
20. a) For distilled water : pH= pOH= [H+]= [OH-]=
b) For 1M HCl: pH= pOH= [H+]= [OH-]=
c) For 1M NaOH: pH= pOH= [H+]= [OH-]=
21. The pH of 0.20 M NaX is 12.50; calculate the Kb.
22. The pH of 0.2 M HX is 4.5; calculate the Ka.
23. 100.0 mL of 0.200 M NaOH is mixed with 100.0 mL of 0.180 M HCl. Calculate the pH of the resulting solution.
24. How many grams of NaHCO3 are required to make 100.0 mL of 0.200M solution?
25. What volume of 0.200 M NaOH is required to neutralize 25.0 mL of 0.150 M H2SO4?
26. In a titration 25.0 mL of .200M H2SO4 is required to neutralize 10.0 mL NaOH. Calculate the concentration of the base.
27. Calculate the concentration of a solution of NaCl made by dissolving 50.0 g in 250.0 mL of water.
28. SO3(g) +
H2O(g) ⇄ H2SO4(l)
Equilibrium concentrations are found to be:
[SO3] = 0.400 M [ H2O] = 0.480 M [H2SO4] = 0.600 M
Calculate the value of the equilibrium constant.
29. 4.00 moles of SO2 and 5.00 moles O2 are placed in a 2.00 L container at 200º C and allowed to reach equilibrium. If the equilibrium concentration of O2 is
2.00 M, calculate the Keq.
2SO2(g) + O2(g) ⇄ 2SO3(g)
Worksheet # 15 Buffers and indicators
Buffers
1. Definition
2.
Acid Conjugate Base Salt
HCN
KHCO3
NH3
HF
NaCH3COO
HC2O4-
3. Write an equation for the first three buffer systems above.
4. Which buffer could have a pH of 4.0? Which buffer could have a pH of 10.0?
a) HCl & NaCl b) HF & NaF c) NH3 & NH4Cl
5. Predict how the buffer of pH = 9.00 will change. Your possible answers are 9.00, 8.98, 9.01, 2.00, and 13.00
Final pH
a) 2 drops of 0.10 M HCl are added
b) 1 drop of 0.10 M NaOH is added
c) 10 mL of 0.10 M HCl are added
6. Write an equation for the carbonic acid, sodium hydrogencarbonate buffer system. A few drops of HCl are added. Describe the shift and each concentration change.
Equation:
Shift [H+] = [H2CO3] = [HCO3-]
=
Indicators
1. Definition
2. Equilibrium equation
3. Colors for methyl orange HInd Ind-
4. Compare the relative sizes of [HInd] and [Ind-] at the following pH’s for methyl orange.
Color Relationship
pH = 2.0
pH = 3.7
pH = 5.0
5. HCl is added to methyl orange, describe if each increases or decreases.
[H+]
[HInd]
[Ind-]
Color Change
6. NaOH is added to methyl orange, describe if each increases or decreases.
[H+]
[HInd]
[Ind-]
Color Change
7. State two equations that are true at the transition point of an indicator.
8. What indicator has a Ka = 4 x 10-8
9. What is the Ka for methyl orange?
10. A solution is pink in phenolphthalein and colorless in thymolphthalein. What is the pH of the solution?
11. A solution is blue in bromothymol blue, red in phenol red, and yellow in thymol blue. What is the pH of the solution?
Worksheet # 16
Titration Curves
Choose an indicator and describe the approximate pH of the equivalence point for each titration. Complete each reaction.
pH Indicator
1. HCl + NaOH →
2. HF + RbOH →
3. HI + Ba(OH)2 →
4. HCN + KOH →
5. HClO4 + NH3 →
6. CH3COOH + LiOH →
7. Calculate the Ka of bromothymol blue.
8. An indicator has a Ka = 1 x 10-10, determine the indicator.
9. Calculate the Ka of methyl orange.
10. An indicator has a Ka = 6.3 x 10-13, determine the indicator.
11. Explain the difference between an equivalence point and a transition point.
Draw a titration curve for each of the following.
12. Adding 100 ml 1.0 M NaOH to 13. Adding 100 ml 1.0 M NaOH to
50 mL 1.0 M HCl 50 mL 1.0 M HCN


pH
Volume of base added Volume of base added
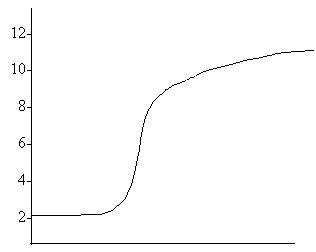
14. Adding 100 ml 0.10 M HCl 15. Adding 100 ml .10 M HCl to 50 mL 0.10M NH3 to 50 mL 0.10 M NaOH
pH pH
Volume of HCl added Volume of HCl added
Acids
Unit Midterm Practice Test
1. Consider the following:
I H2CO3 + F- D
HCO3-
+ HF
II HCO3- + HC2O4- D
H2CO3
+ C2O42-
III HCO3- + H2C6H6O7-
D H2CO3 + HC6H5O72-
The HCO3- is a base in
A.
I only
B.
I and II only
C.
II and III only
D.
I, II, and III
2. Consider the following equilibrium for
an indicator:
HInd + H2O D
Ind- + H3O+
When a few drops of indicator methyl red are added to 1.0 M HCl, the
colour of the resulting solution is
A. red and the products are
favoured
B. red and the reactants are
favoured
C. yellow and the products
are favoured
D. yellow and the reactants
are favoured
3. The volume of 0.200 M Sr(OH)2
needed to neutralize 50.0 mL of 0.200 M HI is
A. 10.0
mL
B. 25.0
mL
C. 50.0
mL
D. 100.0
mL
4. The pOH of 0.050 M HCl is
A. 0.050
B. 1.30
C. 12.70
D. 13.70
5. The volume of 0.450 M HCl needed to
neutralize 40.0 mL of 0.450 M Sr(OH)2 is
A. 18.0
mL
B. 20.0
mL
C. 40.0
mL
D. 80.0
mL
6. Consider the following
I H3PO4 II H2PO4- III HPO42- IV PO43-
Which of the above solutions are
amphiprotic?
A. I
and II only
B. II
and III only
C. I,
II, and III only
D. II,
III, and IV only
7. Which of the following solutions will
have the largest [H3O+]?
A. 1.0
M HNO2
B. 1.0
M HBO3
C. 1.0
M H2C2O4
D. 1.0
M HCOOH
8. Consider the following: H2O + 57
kJ D H3O+ + OH-
When the temperature of the system
is increased, the equilibrium shifts
A. left
and the Kw increases
B. left
and the Kw decreases
C. right
and the Kw increases
D. right
and the Kw decreases
9. Normal rainwater has a pH of
approximately 6 as a result of dissolved
A. oxygen
B. carbon
dioxide
C. sulphur
dioxide
D. nitrogen
dioxide
10. A 1.0 M solution of sodium dihydrogen
phosphate is
A. acidic
and the pH < 7.00
B. acidic
and the pH > 7.00
C. basic
and the pH < 7.00
D. basic
and the pH > 7.00
11. Consider the following equilibrium for an
indicator:
HInd + H2O D
Ind- + H3O+
When a few drops of indicator chlorophenol red are added to a colourless
solution of pH 4.0, the resulting solution is
A. red as [HInd] < [Ind-]
B. red as [HInd] > [Ind-]
C. yellow as [HInd] <
[Ind-]
D. yellow as [HInd] >
[Ind-]
12. A Bronsted-Lowry base is defined as a
chemical species that
A. accepts
protons
B. neutralizes
acids
C. donated
electrons
D. produces
hydroxides ions in solution
13. Which of the following solutions will
have the greatest electrical conductivity?
A. 1.0
M HCN
B. 1.0
M H2SO4
C. 1.0
M H3PO4
D. 1.0
M CH3COOH
14. Consider the following equilibrium: HC6H5O72- +
HIO3 D H2C6H5O7- + IO3-
The order of Bronsted-Lowry acids
and bases is
A. acid,
base, acid, base
B. acid,
base, base, acid
C. base,
acid, acid, base
D. base,
acid, base acid
15. Consider the following: H2O(l) D
H+ + OH-
When a small amount of 1.0 M KOH is added to the above system, the
equilibrium
A. shifts left and [H+]
decreases
B. shifts left and [H+]
increases
C. shifts right and [H+]
decreases
D. shifts right and [H+]
increases
16. Which of the following has the highest
pH?
A. 1.0
M NaIO3
B. 1.0
M Na2CO3
C. 1.0
M Na3PO4
D. 1.0
M Na2SO4
17. In a 100.0 mL sample of 0.0800 M NaOH the
[H3O+] is
A. 1.25 x 10-13
M
B. 1.25 x 10-12
M
C. 8.00 x 10-3
M
D. 8.00 x 10-2
M
18. Consider the following:
I ammonium nitrate II calcium
nitrate III iron III nitrate
When dissolved in water, which of these salts would form a neutral
solution?
A. II only
B. III only
C. I and III only
D. I, II, and III
19. Consider the following: SO42- +
HNO2 D HSO4- + NO2-
Equilibrium would favour the
A. the
products since HSO4- is a weaker acid than HNO2
B. the
reactants since HSO4- is a weaker acid than HNO2
C. the
products since HSO4- is a stronger acid than HNO2
D. the
reactants since HSO4- is a stronger acid than HNO2
20. The net ionic equation for the hydrolysis
of Na2CO3 is
A. H2O + Na+ D
NaOH + H+
B. H2O +
2Na+ D Na2O + 2H+
C. H2O + CO32- D
H2CO3
+ O2-
D. H2O + CO32- D
HCO3-
+ OH-
21. Consider the following equilibrium: 2H2O(l) D
H3O+ + OH-
A few drops of 1.0 M HCl are added
to the above system. When equilibrium is
re-established, the
A. [H3O+]
has increased and the [OH-] has decreased
B. [H3O+]
has increased and the [OH-] has increased
C. [H3O+]
has decreased and the [OH-] has increased
D. [H3O+]
has decreased and the [OH-] has decreased
22. A basic solution
A. tastes
sour
B. feels
slippery
C. does
not conduct electricity
D. reacts
with metals to release oxygen gas
23. The balanced formula equation for the
neutralization of H2SO4 by KOH is
A. H2SO4 +
KOH → KSO4 + H2O
B. H2SO4 +
KOH → K2SO4 + H2O
C. H2SO4 +
2KOH → K2SO4 + H2O
D. H2SO4 +
2KOH → K2SO4 + 2H2O
24. An Arrhenius base is defined as a
substance which
A. donates
protons
B. donates
electrons
C. produces
H+ in solution
D. produces
OH- in solution
25. Consider the following equilibrium: HS- + H3PO4 D
H2S + H2PO4-
The order of Bronsted-Lowry acids
and bases is
A. acid,
base, acid, base.
B. acid,
base, base, acid
C. base,
acid, acid, base
D. base,
acid, base, acid
26. The equation representing the reaction of
ethanoic acid with water is
A. CH3COO- + H2O D
CH3COOH + OH-
B. CH3COO- + H2O D
CH3COO2-
+ H3O+
C. CH3COOH + H2O D
CH3COO-
+ H3O+
D. CH3COOH + H2O D
CH3COOH2+ + OH-
27. Consider the following equilibrium: 2H2O + 57kJ D
H3O+
+ OH-
When the temperature is decreased,
the water
A. stays
neutral and the [H3O+] increases
B. stays
neutral and the [H3O+] decreases
C. becomes
basic and [H3O+] decreases
D. becomes
acidic and [H3O+] increases
28. The equation for the reaction of Cl2O
with water is
A. Cl2O + H2O D
2HClO
B. Cl2O + H2O D
2ClO + H2
C. Cl2O + H2O D
Cl2 + H2O2
D. Cl2O + H2O D
Cl2 + O2 + H2
29. The conjugate acid of C6H50-
is
A. C6H4O-
B. C6H5OH
C. C6H4O2-
D. C6H5OH+
30. Which of the following solutions will
have the greatest electrical conductivity?
A. 1.0
M HCl
B. 1.0
M HNO2
C. 1.0 M H3BO3
D. 1.0 M HCOOH
31. A solution of 1.0 M HF has
A. a
lower pH than a solution of 1.0 M HCl
B. a
higher pOH than a solution of 1.0 M HCl
C. a
higher [OH-] than a solution of 1.0 M HCl
D. a
higher [H3O+] than a solution of 1.0 M HCl
32. Which of the following is the weakest
acid
A. HIO3
B. HCN
C. HNO3
D. C6H5COOH
33. Considering the following data
H3AsO4 Ka
= 5.0 x 10-5
H2AsO4- Ka
= 8.0 x 10-8
HAsO42- Ka
= 6.0 x 10-10
The Kb value for H2AsO4- is
A. 2.0 x 10-10
B. 8.0 x 10-8
C. 1.2 x 10-7
D. 1.7 x 10-5
34. In a solution at 25oC, the [H3O+]
is 3.5 x 10-6 M. The [OH-] is
A. 3.5 x 10-20
M
B. 2.9 x 10-9
M
C. 1.0 x 10-7
M
D. 3.5 x 10-6
M
35. In a solution with a pOH of 4.22, the
[OH-] is
A. 1.7 x 10-10
M
B. 6.0 x 10-5
M
C. 6.3 x 10-1
M
D. 1.7 x 104
M
36. An aqueous solution of NH4CN
is
A. basic
because Ka < Kb
B. basic
because Ka > Kb
C. acidic
because Ka < Kb
D. acidic
because Ka > Kb
37. The net ionic equation for the
predominant hydrolysis reaction of KHSO4 is
A. HSO4- + H2O
D SO42- + H3O+
B. HSO4- + H2O D
H2SO4
+ OH-
C. KHSO4 + H2O D
K+ + SO42- + H3O+
D. KHSO4 + H2O D
K+ + H2SO4 + OH-
38. The [OH-] in an aqueous
solution always equals
A. Kw x [H3O+]
B. Kw - [H3O+]
C. Kw/[H3O+]
D. [H3O+]/Kw
39. The [H3O+] in a
solution with pOH of 0.253 is
A. 5.58 x 10-15
M
B. 1.79 x 10-14
M
C. 5.58 x 10-1
M
D. 5.97 x 10-1
M
40. The equilibrium expression for the
hydrolysis reaction of 1.0 M K2HPO4 is
A. [H2PO4-][OH-] B. [H3PO4][OH-]
[HPO42-] [H2PO4-]
C. [K+] [KHPO4-] D. [K+]2
[HPO42-]
[K2HPO4] [K2HPO4]
41. The solution with the highest pH is
A. 1.0
M NaCl
B. 1.0
M NaCN
C. 1.0
M NaIO3
D. 1.0
M Na2SO4
42. The pH of 100.0 mL of 0.0050 M NaOH is
A. 2.30
B. 3.30
C. 10.70
D. 11.70
43. Consider the following equilibrium for an
indicator: HInd + H2O D Ind- + H3O+
At the transition point,
A. [HInd] >
[Ind-]
B. [HInd] =
[Ind-]
C. [HInd] <
[Ind-]
D. [HInd] = [H3O+]
Acids
Unit Midterm Practice Test Subjective
1. a) Write
the net ionic equation for the reaction between NaHSO3 and NaHC2O4.
b) Explain
why the reactants are favoured in the above reaction.
2. What
is the [H3O+] in a solution formed by adding 60.0 mL of
water to 40.0 mL
of
0.400 M KOH?
3. A solution of 0.100 M HOCN has a pH of
2.24. Calculate the Ka value for the acid.
4. Calculate the pH in 100.0 mL 0.400 M H3BO3.
5. Calculate
the pH of the solution formed by mixing 20.0 mL of 0.500 M HCl with 30.0 mL of 0.300 M NaOH.
6. a) Write
the balanced equation representing the reaction of HF with H2O.
b) Identify
the Bronsted-Lowry bases in the above equation.
7. Consider the following data:
Barbituric acid HC4H3N2O3 Ka = 9.8 x 10-5
Sodium propanoate NaC3H5O2 Kb = 7.5 x 10-10
Propanoic acid HC3H5O2 Ka = ?
Which is the stronger acid,
propanoic acid or babituric acid? Explain using calculations.
8. A solution of 0.0100 M lactic acid, HC3H5O3,
has a pH of 2.95. Calculate the Ka value.
9. a) Write
equations showing the amphiprotic nature of water as it reacts with HCO3-.
b) Calculate
the Kb for HCO3-.
10. Calculate the [H3O+]
in 0.550 M C6H5COOH.
Quiz #1
Properties of Acids, Bases, Salts, Arrhenius Bronsted Acids, Ka,
Strength
1. Drano®, a commercial product used to clean drains, contains small bits of aluminum metal and
A. ammonia
B. acetic acid
C. hydrochloric acid
D. sodium hydroxide
2. A net ionic equation for the reaction between CH3COOH and KOH is
A. CH3COOH(aq) + K+(aq) ⇄ CH3COOK(aq)
B. CH3COOH(aq) + OH-(aq) ⇄ H2O(l) + CH3COO-(aq)
C. CH3COOH(aq) + KOH(aq) ⇄ H2O(l) + CH3COOK(aq)
D. CH3COOH(aq) + K+(aq) + OH-(aq)⇄ H2O(l) + KCH3COO(aq)
3. Which equation represents a neutralization reaction?
A. Pb2+(aq) + 2Cl-(aq) → PbCl2(s)
B. HCl(aq) + NH3(aq) → NH4Cl(aq)
C. BaI2(aq) + MgSO4(aq) → BaSO4(s) + MgI2(aq)
D. MnO4-(aq) + 5Fe2+(aq) +8H+(aq) → Mn2+(aq) + 5Fe3+(aq) + 4H2O(l)
4. An Arrhenius acid is a substance that
A. accepts a proton
B. donates a proton
C. produces H+ in solution
D. produces OH- in solution
5. Consider the following data table:
|
Breaker |
Volume |
Contents |
|
1 |
15 mL |
0.1 M Sr(OH)2 |
|
2 |
20 mL |
0.2 M NH4OH |
|
3 |
25 mL |
0.1 M KOH |
|
4 |
50 mL |
0.2 M NaOH |
Identify the beaker that requires the smallest volume of 1.0 M HCl for complete neutralization
A. Beaker 1
B. Beaker 2
C. Beaker 3
D. Beaker 4
6. The net ionic equation for the titration of HClO4(aq) with LiOH(aq) is
A. H+(aq) + OH-(aq) → H2O(l)
B. HClO4(aq) + OH-(aq) → ClO4-(aq) + H2O(l)
C. HClO4(aq) + LiOH(aq) → LiClO4(aq) + H2O(l)
D. H+(aq) + ClO4-(aq) + Li+(aq) + OH-(aq) → LiClO4(aq) + H2O(l)
7. The equilibrium constant expression for a sulphurous acid is
A Ka = [H+][HSO3-]
B. Ka = [H+][HSO3-]
[H2SO3]
C. Ka = [2H+][SO32-]
[H2SO3]
D. Ka = [H+][SO32-]
[H2SO3]
8. To distinguish between a strong acid and a strong base, an experimenter could use
A. odor
B. magnesium
C. a conductivity test
D. the common ion test
9. How many acids from the list below are known to be weak acids?
HCl, HF, H2SO3, H2SO4, HNO3, HNO2
A. 2
B. 3
C. 4
D. 5
10. There are two beakers on a laboratory desk. One beaker contains 1.0 M HCl and the other contains tap water. To distinguish the acid solution from the water, one would use
A. a piece of copper.
B. a piece of magnesium
C. phenolphthalein indicator
D. a piece or red litmus paper
11. Caustic soda, NaOH, is found in
A. fertilizers
B. beverages
C. toothpaste
D. oven cleaners
12. Which of the following is the strongest acid?
A. Acetic acid
B. Oxalic acid
C. Benzoic acid
D. Carbonic acid
13. The acid used in the lead-acid storage battery is
A. HCl
B. HNO3
C. H2SO4
D. CH3COOH
Quiz #2 Conjugates, Amphiprotic, Arrhenius, Bronsted
Bases, Kb, & Strength
1. A test that could be safely used to distinguish a strong base from a weak base is
A. taste
B. touch
C. litmus paper
D. electrical conductivity
2. Identify the two substances that act as Bronsted-Lowry bases in the equation
HS- + SO42- ⇄ S2- + HSO4-
A. HS-
and S2-
B. SO4 2-and S2-
C. HS- and HSO4-
D. SO42- and HSO4-
3. The conjugate acid of H2C6H5O-7 is
A. C6H5O73-
B. HC6H5O72-
C. H2C6H5O7
D. H3C6H5O7
4. Which one of the following substances is/are amphiprotic?
(1) H3PO4 (2) H2PO4- (3) HPO42-
A. 2 only
B. 3 only
C. 1 and 2
D. 2 and 3
5. The net ionic equation for the neutralization of HBr by Ca(OH)2 is
A. H+(aq) + OH-(aq) ⇄ H2O(l)
B. Ca2+(aq) + 2Br-(aq) ⇄ CaBr2(s)
C. 2HBr(aq) + Ca(OH)2(aq) ⇄ 2H2O(l) + CaBr2(s)
D. 2H+(aq) + 2Br -(aq) + Ca2+(aq) + 2OH-(aq) ⇄ 2H2O(l) + Ca2+(aq) + 2Br -(aq)
6. If reactants are favored in the following equilibrium, the stronger base must be
HCN + HS - ⇄ H2S + CN -
A. H2S
B. HS-
C. CN-
D. HCN
7. The hydronium ion, H3O+ is a water molecule that has
A. lost a proton
B. gained a proton
C. gained a neutron
D. gained an electron
8. The complete ionic equation for the neutralization of acetic acid by sodium hydroxide is
A. H+ + OH- ⇄ H2O
B. CH3COOH + OH- ⇄ CH3COO- + H2O
C. CH3COOH + NaOH ⇄ NaCH3COOH + H2O
D. CH3COOH + Na+ + OH- ⇄ Na+ + CH3COO- + H2O
9. In the following Bronsted – Lowry acid-base equation:
NH4+ (aq) + H2O(l) ⇄ NH3(aq) + H3O+(aq)
The stronger base is
A. NH4+
B. H2O
C. NH3
D. H3O+
10. Consider the following equilibrium system:
OCl-(aq) + HC7H5O2(aq) ⇄ HOCl(aq) + C7H5O2-(aq) Keq= 2.1 x 103
At Equilibrium
A. products are favored and HOCl is the stronger acid
B. reactants are favored and HOCl is the stronger acid
C. products are favored and HC7H5O2 is the stronger acid
D. reactants are favored and HC7H5O2 is the stronger acid
11. In the equilibrium system
H2BO3- (aq) + HCO3-(aq) ⇄ H2CO3(aq) + HBO32-(aq)
The two species acting as Bronsted-Lowry acids are
A. HCO3- and H2CO3
B. H2BO3- and H2CO3
C. HCO3- and HBO32-
D. H2BO3- and HBO32-
12. Consider the following equilibrium HS- + H2C2O4 ⇄ HC2O4- + H2S
The stronger acid is
A. HS-
B. H2C2O4
C. HC2O4-
D. H2S
Quiz #3 Leveling effect, Anhydrides, Hydrolysis, Relationships
1. Which of the following oxides will form the most acidic solution?
A. SO2
B. MgO
C. Na2O
D. Al2O3
2. Which one of the following salts will produce an acidic solution?
A. KBr
B. LiCN
C. NH4Cl
D. NaCH3COO
3. The balanced equation for the reaction between sodium oxide and water is
A. Na2O + H2O → 2NaOH
B. Na2O + H2O → 2NaH + O2
C. Na2O + H2O →
2Na + H2O2
D. Na2O + H2O → 2Na + H2 +O2
4. ‘Normal’ rainwater is slightly acidic due to the presence of dissolved
A. methane
B. carbon dioxide
C. sulphur dioxide
D. nitrogen dioxide
5. Which of the following oxides would hydrolyze to produce hydroxide ions?
A. NO
B. SO2
C. Cl2O
D. Na2O
6. The approximate pH of “normal” rainwater is
A. 0
B. 6
C. 7
D. 8
7. Which of the following oxides would hydrolyze to produce hydronium ions?
A. CaO
B. SO2
C. MgO
D. Na2O
8. Which of the following gasses results in the formation of acid rain?
A. H2
B. O3
C. SO2
D. NH3
9. Consider the following acid base solution
HSO3- + HF ⇄ H2SO3 + F-
The order of Bronsted-Lowry acids and bases in this equation is
A. acid + base ⇄ acid + base
B. acid + base ⇄ base + acid
C. base + acid ⇄ base + acid
D. base + acid ⇄ acid + base
10. The conjugate acid of OH- is
A. H+
B. O2-
C. H2O
D. H3O+
11. Which of the following 0.10 M solutions will have the greatest electrical conductivity?
A. HF
B. NH3
C. NaOH
D. C6H5COOH
12. The amphiprotic ion HSeO3- can undergo hydrolysis according to the following equations
|
HSeO3- + H2O ⇄ H2SeO3 + OH- |
K1 |
|
HSeO3- + H2O ⇄ SeO32-+ H3O+ |
K2 |
An aqueous solution of HSeO3- is found to be acidic. This observation indicates that when it is added to water, HSeO3- behaves mainly as a
A. proton donor, and K1 is less than K2
B. proton donor, and K1 is
greater than K2
C. proton acceptor, and K1 is less than K2
D. proton acceptor, and K1 is
greater than K2
13. The Kb expression for HPO42- is
A. [PO43-][H3O+]
B. [HPO42-][OH-]
[HPO42-] [H2PO4-]
C. [H2PO4-][OH-] D. [HPO42-][
H3O+]
[HPO42-] [PO43-]
Quiz #4 Anhydrides,
Hydrolysis
1. Which of the following pairs of gases are primarily responsible for producing “acid rain”?
A. O2 and O3
B. N2 and O2
C. CO and CO2
D. NO2 and SO2
2. Sodium potassium tartrate (NaKC4H4O6) is used to raise the pH of fruit during processing. In this process, sodium potassium tartrate is being used as a/an
A. salt
B. acid
C. base
D. buffer
3. The net ionic equation for the hydrolysis of the salt, Na2S is
A. Na2S ⇄ 2Na+ + S2-
B. S2- + H2O ⇄ OH- + HS-
C. Na2S + 2H2O ⇄ 2NaOH + H2S
D. 2Na+ + S2- + 2H2O ⇄ 2Na+ + 2OH- + H2S
4. Which of the following solutions would be acidic?
A. sodium acetate
B. iron III chloride
C. sodium carbonate
D. potassium chloride
5. Consider the following salts: I. NaF II. NaClO4 III. NaHSO4
Which of these salts, when dissolved in water, would form a basic solution?
A. I only
B. I and II only
C. II and III only
D. I, II and III
6. Which of the following, when dissolved in water, forms a basic solution?
A. KCl
B. NaClO4
C. Na2CO3
D. NH4NO3
7. Which of the following oxides forms a basic solution?
A. K2O
B. CO2
C. SO3
D. NO2
8. Which of the following is amphiprotic in water?
A. SO2
B. SO32-
C. HSO3-
D. H2SO3
9. Consider the following equilibrium expression
K= [H2S][OH-]
[HS-]
This expression represents the
A. Kb for H2S
B. Ka for H2S
C. Kb for HS-
D. Ka for HS-
10. The reaction of a strong acid with a strong base produces
A. A salt and a water
B. A base and an acid
C. A metallic oxide and water
D. A non-metallic oxide and water
11. Consider the following equilibrium:
CH3COOH(aq) + NH3(aq) ⇄ CH3COO- (aq) + NH4+(aq)
The sequence of Bronsted-Lowry acids and bases in the above equilibrium equation is
A. acid, base, base, acid
B. acid, base, acid, base
C. base, acid, base, acid
D. base, acid, acid, base
12. The pH range of ‘acid rain’ is often
A. 3 to 6
B. 6 to 8
C. 7 to 9
D. 10 to 12
13. Water will act as a Bronsted-Lowry acid with
A. NH3
B. H2S
C. HCN
D. HNO3
14. Which of the following is a conjugate acid-base pair?
A. H3PO4 and PO43-
B. H2PO4- and PO43-
C. H3PO4 and HPO42-
D. H2PO4- and HPO42-
Quiz #5 pH calculations for Strong and
Weak Acids
1. The 1.0 M acidic solution with the highest pH is
A. H2S
B HNO2
C. HNO3
D. H3BO3
2. At 25oC, the equation representing the ionization of water is
A H2O + H2O ⇄ 2H2 + O2
B. H2O + H2O ⇄ H2O2 + H2
C. H2O + H2O ⇄ 4H+ + 2O2-
D. H2O + H2O ⇄ H3O+ +OH-
3. The pH of a 0.3 M solution of NH3 is approximately
A. 14.0
B. 11.0
C. 6.0
D. 3.0
4. The pH of an aqueous solution is 4.32. The [OH-] is
A. 6.4 x 10-1 M
B. 4.8 x 10-5 M
C. 2.1 x 10-10 M
D. 1.6 x 10-14 M
5. The pH of an aqueous solution is 10.32. The [OH-] is
A. 5.0 x 10-12 M
B. 2.0 x 10-11 M
C. 4.8 x 10-11 M
D. 2.1 x 10-4 M
6. The pH of a 0.025 M HClO4 solution is
A. 0.94
B. 1.60
C. 12.40
D. 13.06
7. Consider the following equilibrium: H2O(l) + H2O(l) ⇄ H3O+(aq) + OH-(aq)
The equilibrium constant for this system is referred to as
A. Kw
B. Ka
C. Kb
D. Ksp
8. The [H3O+] in a solution of pH = 0.60 is
A. 4.0 x 10-14 M
B. 2.2 x 10-1 M
C. 2.5 x 10-1 M
D. 6.0 x 10-1 M
9. A solution is prepared by adding 100 mL of 10 M of HCl to a 1 litre volumetric flask and filling it to the mark with water. The pH of this solution is
A. -1
B. 0
C. 1
D. 7
10. The approximate pH of a 0.06 M solution of CH3COOH is
A. 1
B. 3
C. 11
D. 13
11. The [OH-] is greater than the [H3O+] in
A. HCl(aq)
B. NH3(aq)
C. H2O(aq)
D. CH3COOH(aq)
12. The pH of 0.15 M HCl is
A. 0.15
B. 0.71
C. 0.82
D. 13.18
13. Which of the following equations correctly relates pH and [H3O+]?
A. pH= log [H3O+]
B. pH= 14 - [H3O+]
C. pH= -log [H3O+]
D. pH= pKw – [H3O+]
14. The pH of 0.20 M HNO3 is
A. 0.20
B. 0.63
C. 0.70
D. 1.58
15. The [OH-] in 0.050 M HNO3 at 25oC is
A. 5.0 x 10-16 M
B. 1.0 x 10-14 M
C. 2.0 x 10-13 M
D. 5.0 x 10-2 M
Quiz #6 Ka’s from pH Kb’s from Ka’s
1. The Kb for the dihydrogen phosphate ion is
A. 1.3 x 10-12
B. 6.3 x 10-8
C. 1.6 x 10-7
D. 7.1 x 10-3
2. What volume of 0.100 M NaOH is required to neutralize a 10.0 mL sample of 0.200 M H2SO4?
A. 5.0 mL
B. 10.0 mL
C. 20.0 mL
D. 40.0 mL
3. Consider the following equilibriums:
|
I |
HCO3- + H2O ⇄ H2CO3 + OH- |
|
II |
NH4+ + H2O ⇄ H3O+ + NH3 |
|
III |
HSO3- + H3O+ ⇄ H2O + H2SO3 |
Water acts as a Bronsted-Lowry base in
A. III only
B. I and II only
C. II and III only
D. I, II, and III
4. Which of the following is represented by a Kb expression?
A. Al(OH)3(s) ⇄ Al3+(aq) + 3OH-(aq)
B. HF(aq) + H2O(l) ⇄ H3O+(aq) + F-(aq)
C. Cr2O72-(aq) + 2OH-(aq) ⇄ 2CrO42-(aq) + H2O(l)
D. CH3NH2(aq) + H2O(l) ⇄ CH3NH3+ (aq) + OH-(aq)
5. A student combines 0.25 mol of NaOH and 0.20 mol of HCl in water to make 2.0 liters of solution. The pH of the solution is
A. 1.3
B. 1.6
C. 12.4
D. 12.7
6. If OH- is added to a solution, the [H3O+] will
A. Remain constant
B. Adjust such that [H3O+]= [OH-]
Kw
C. Increase such that [H3O+][OH-] = Kw
D. Decrease such that [H3O+][OH-] = Kw
7. In a titration, 10.0 mL of H2SO4(aq) is required to neutralize 0.0400 mol of NaOH.
From this data, the [H2SO4] is
A. 0.0200 M
B. 2.00 M
C. 4.00 M
D. 8.00 M
8. Consider the following equilibrium for an acid-base indicator:
Hlnd ⇄ H+ + Ind- Ka = 1.0 x 10-10
Which of the following statements is correct at pH 7.0?
A. [Ind-] < [HInd]
B. [Ind-] = [HInd]
C. [Ind-] > [HInd]
D. [Ind-] = [H+] = [HInd]
9. Which of the following indicators would be yellow at pH 7 and blue at pH 10?
A. thymol blue
B. methyl violet
C. bromthymol blue
D. bromcresol green
10. Consider the following equilibrium for phenolphthalein: HInd ⇄ H+ + Ind-
When phenolphthalein is added to 1.0 M NaOH, the color of the resulting solution is
A. pink as [HInd] is less than [Ind-]
B. pink as [HInd] is greater than [Ind-]
C. colorless as [HInd] is less than [Ind-]
D. colorless as [HInd] is greater than [Ind-]
11. Water acts as a base when it reacts with
A. CN-
B. NH3
C. NO2-
D. NH4+
12. What is the pH of a solution prepared by adding 0.50 mol KOH to 1.0 L of
0.30 M HNO3?
A. 0.20
B. 0.70
C. 13.30
D. 13.80
13. The 1.0 M acid solution with the largest [H3O+] is
A. HNO2
B. H2SO3
C. H2CO3
D. H3BO3
Quiz #7 pH for Weak bases, pH Relationships,
Amphiprotic Calculations
1. In water, the hydrogen sulphide ion, HPO42-, will act as
A. An acid because Ka < Kb
B. An acid because Ka > Kb
C. A base because Ka < Kb
D. A base because Ka > Kb
2. A student records the pH of 1.0 M solution of two acids. Which of the following statements can be concluded from the above data?
|
Acid |
pH |
|
X |
4.0 |
|
Y |
2.0 |
A. Acid X is stronger than acid Y
B. Acid X and acid Y are both weak
C. Acid X is diprotic while acid Y is monoprotic
D. Acid X is 100 times more concentrated than acid Y
3. When added to water, the hydrogen carbonate ion, HCO3-, produces a solution, which is
A. basic because Kb is greater than Ka
B. basic because Ka is greater than Kb
C. acidic because Ka is greater than Kb
D. acidic because Kb is greater than Ka
4. The concentration, Ka and pH values of four acids are given in the following table
|
ACID |
Concentration |
Ka |
pH |
|
HA |
3.0 M |
2.0 x 10-5 |
2.1 |
|
HB |
0.7 M |
4.0 x 10-5 |
2.3 |
|
HC |
4.0 M |
1.0 x 10-5 |
2.2 |
|
HD |
1.5 M |
1.3 x 10-5 |
2.4 |
Based on this data, the strongest acid is
A. HA
B. HB
C. HC
D. HD
5. Which of the following 0.10 M solutions is the most acidic?
A. AlCl3
B. FeCl3
C. CrCl3
D. NH4Cl
6. Which of the following acid-base indicators has a transition point between pH 7 and pH 9?
A. Ethyl red, Ka = 8 x 10-2
B. Congo red, Ka = 9.0 x 10-3
C. Cresol red, Ka = 1.0 x 10-8
D. Alizarin blue, Ka = 7.0 x 10-11
Quiz #8 Buffers and Indicators
1. Consider the following acid solutions:
I. H2CO3 II. HClO4 III. HF
Which of the above acids would form a buffer solution when its conjugate base is added?
A. I only
B. II only
C. I and III only
D. I, II, and III only
2. Consider the following base indicator:
HInd ⇄ H+ + Ind-
When the indicator is added to different solutions, the following data are obtained:
|
Solution |
1.0 M HCl |
1.0 M HAl |
1.0 M HA2 |
|
Colour |
Yellow |
Blue |
Yellow |
The acids HAl, HA2, and HInd listed in the order of decreasing acid strength is
A. HA2, HInd, HAl
B. HInd, HAl, HA2
C. HA2, HAl, HInd
D. HAl, HInd, HA2
3. Which of the following compounds, when added to a solution of ammonium nitrate, will result in the formation of a buffer solution?
A. Ammonia
B. Nitric acid
C. Sodium nitrate
D. Ammonium chloride
4. Which of the following represents a buffer equilibrium?
A. HI + H2O ⇄ H3O+ + I-
B. HCl + H2O ⇄ H3O + Cl-
C. HCN + H2O ⇄ H3O+ + CN-
D. HClO4 + H2O ⇄ H3O + ClO4-
5. Consider the following equilibrium:
HF(aq) + H2O(l) ⇄ H3O+(aq) + F-(aq)
The above system will behave as a buffer when the [F-] is approximately equal to
A. Ka
B. [HF]
C. [H2O]
D. [H3O+]
6. A basic buffer solution can be prepared by mixing equal numbers of moles of
A. NH4CL and HCl
B. NaCl and NaOH
C. Na2CO3 and NaHCO3
D. NaCH3COOH and CH3COOH
Quiz #9 Titrations
and Titration Curves
1. Which of the following indicators would be used when titrating a weak acid with a strong base?
A. Methyl red
B. Methyl violet
C. Indigo carmine
D. Phenolphthalein
2. Which of the following acid-base pairs would result in an equivalence point with pH greater than 7.0?
A. HCl and LiOH
B. HNO3 and NH3
C. HClO4 and NaOH
D. CH3COOH and KOH
3. Which of the following standardized solutions should a chemist select when titrating a 25.00 mL sample of 0.1 M NH3, using methyl red as an indicator?
A. 0.114 M HCl
B. 6.00 M HNO3
C. 0.105 M NaOH
D. 0.100 M CH3COOH
4. Consider the following 0.100 M solutions I. H2SO4 II. HCl III. HF
The equivalence point is reached when 10.00 mL of 0.100 NaOH has been added to 10.00 mL of solution(s)
A. II only
B. I and II only
C. II and III only
D. I, II and III
5.


Which pair of 0.10 M solutions would result in the above titration curve?
A. HF and KOH
B. HCl and NH3
C. H2S and NaOH
D. HNO3 and KOH
6. A suitable indicator for the above titration is
A. Methyl violet
B. Alizarin yellow
C. Thymolphthalein
D. Bromcresol green
7. The pH scale is
A. direct
B. inverse
C. logarithmic
D. exponential
8.
Which of the following indicators should be used in the titration represented by the above titration curve?
A. Orange IV
B. Methyl red
C. Phenolphthalein
D. Alizarin yellow
9. Which of the following indicators should be used when 1.0 M HNO2 is titrated with NaOH(aq)?
A. Methyl red
B. Thymol blue
C. Methyl orange
D. Indigo carmine
10. Which of the following solutions should be used when titrating a 25.00 mL sample of CH3COOH that is approximately 0.1 M?
A. 0.150 M NaOH
B. 0.001 M NaOH
C. 3.00 M NaOH
D. 6.00 M NaOH
11. What volume of 0.250 M H2SO4 is required to neutralize 25.00 mL of 2.50 M KOH?
A. 125 mL
B. 150 mL
C. 250 mL
D. 500mL
12. Which of the following pairs of substances form a buffer system for human blood?
A. HCl and Cl-
B. NH3 and NH2-
C. H2CO3 and HCO3-
D. H2C6H5O7 and HC6H5O72-
Quiz #10 Review
1. How many moles of Mg(OH)2 are required to neutralize 30.00 mL of 0.150 M HCl?
A. 2.25 x 10-3 mol
B. 4.50 x 10-3 mol
C. 5.00 x 10-3 mol
D. 9.00 x 10-3 mol
2. The approximate Ka for the indicator phenolphthalein is
A. 6 x 10-19
B. 8 x 10-10
C. 6 x 10-8
D. 2 x 10-2
3. A new indicator, “B.C. Blue (HInd),” is red in bases and blue in acids. Describe the shift in equilibrium and the resulting color change if 1.0 M HIO3 is added to a neutral, purple solution of this indicator: HInd + H2O ⇄ H3O+ + Ind-
A. Equilibrium shifts left, and colour becomes red
B Equilibrium shifts left, and colour becomes blue
C. Equilibrium shifts right, and colour becomes red
D. Equilibrium shifts right, and colour becomes blue
4. Which one of the following combinations would act as an acid buffer?
A. HCl and NaOH
B. KOH and KBr
C. NH3 and NH4Cl
D. CH3COOH and NaCH3COO
5. What is the pH at the transition point of an indicator if its Ka is 7.9 x 10-3?
A. 0.98
B. 2.10
C. 7.00
D. 11.90
6. Which of the following pH curves best represents the titration of sodium hydroxide with hydrochloric acid?
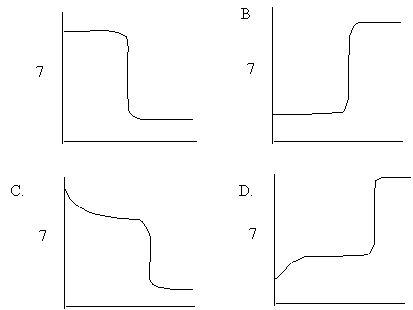
A.
7. A student prepares a buffer by placing ammonium chloride in a solution of ammonia. Equilibrium is established according to the equation: NH3 + H2O ⇄ NH4+ + OH-
When a small amount of base is added to the buffer, the base reacts with
A. NH3 and the pH decreases
B. NH4+ and the pH decreases
C. NH3 to keep the pH relatively constant
D. NH4+ to keep the pH relatively constant
8. At the equivalence point in a titration involving 1.0 M solutions, which of the following combinations would have the lowest conductivity?
A. Nitric acid and barium hydroxide
B. Acetic acid and sodium hydroxide
C. Sulphuric acid and barium hydroxide
D. Hydrochloric acid and sodium hydroxide
9. An indicator HInd produces a yellow colour in 0.1 M HCl solution and a red colour in 0.1 M HCN solution. Therefore, the following equilibrium:
HCN + Ind- ⇄ HInd + CN-
A. Products are favored and the stronger acid is HInd
B. Products are favored and the stronger acid is HCN
C. Reactants are favored and the stronger acid is HInd
D. Reactants are favored and the stronger acid is HCN
10. The indicator methyl red is red in a solution of NaH2PO4. Which of the following equations is consistent with this observation?
A. H2PO4-
+ H2O ⇄
HPO42- + H3O+
B. H2PO4-
+ H2O ⇄
H3PO4 + OH-
C. HPO42- + H2O ⇄ PO43- + H3O+
D. HPO42- + H2O ⇄ H2PO4- + OH-
11. Consider the following acid-base indicator equilibrium:
HInd(aq) + H2O(l) ⇄ H3O+(aq) ⇄ Ind-(aq)
Which of the following statements describes the conditions that exist in an indicator equilibrium system at its transition point?
A. [HInd] = [Ind-]
B. [Ind-] = [H3O+]
C. [HInd] = [H3O+]
D. [H3O+] = [H2O]
12. Which of the following titrations would have an equivalence point less that pH 7?
A. NH3 and HCl
B. NaOH and HNO3
C. Ba(OH)2 and H2SO4
D. KOH and CH3COOH
Web Review of Acids
1. List five properties of:
a) acids
b) bases
2. What ion is produced when an acid reacts with water? A base?
3. Define:
Conjugate
Arhenius strong acid
Bronsted weak acid
Bronsted strong base
Ionization of water
Equivalence point
Transition point
Buffer
Hydrolysis.
4. Identify the acids or bases in the following equation. Are the reactants or products favoured?
HC03- + HF ⇄ H2CO3 + F-
5. Classify each compound as a strong or weak acid or base; acidic or basic anhydride; acidic, basic, or neutral salt; or buffer system. Write an equation to show how each reacts with water.
|
NH3 |
AlCl3 |
H2CO3 |
HClO4 |
KCN |
NH4Cl |
KOH |
|
SO2 |
NaF |
HCl |
NaI |
K2O |
NaOH |
CO2 |
|
NH3 and NH4Cl |
NaCH3COO and CH3COOH |
6. H+is short for _______.
7. Determine the conjugates for each of the bases.
|
CN- |
NH3 |
F- |
OH- |
Co(H2O)5(OH)2+ |
8. Determine the conjugates for each of the acids.
|
HF |
HCN |
Al(H2O)63+ |
NH4+ |
HPO42- |
9. Describe a strong and weak acid as well as a strong and weak base in terms of each of the following:
strong acid weak acid strong base weak base
Conductivity
Size of Ka
Size of Kb
Degree of Ionization
pH.
10. Why is the strongest acid in water H3O+? Explain!
11. Why is the strongest base in water OH-? Explain!
12. Which has the higher pH H2S03 or H3BO3? Explain!
13. Which has the higher pH NaCN or NaF? Explain!
14. A buffer has a pH of 9.00. 2 drops of a dilute strong acid are added. Estimate how the pH changes?
15. a) Complete the chart by indicating the pairs required to make buffer solutions. For example HCN (weak acid) and NaCN (salt containing the conjugate of the weak acid) will make a buffer solution. b) Write an equation the describes the equilibrium for each buffer. c) Circle the formulas that have high concentrations.
|
Weak Acid or Base |
Salt |
|
HF |
|
|
|
NaCH3COO |
|
NH3 |
|
|
|
NaCN |
|
H2CO3 |
|
|
|
KH2PO4 |
|
HCH3COO |
|
16. Match each equation with its type:
|
Acid/base formula equation |
F- + HOH(l) ⇄ HF + OH- |
|
Acid/base net ionic equation |
HCl + NaOH →NaCl + HOH(l) |
|
Solubility product ionization equation |
H+ + OH- → HOH(l) |
|
Hydrolysis of a weak acid |
AgCl(s) ⇄ Ag+ + Cl- |
|
Hydrolysis of a weak base |
H20(l) ⇄ H+ + OH- |
|
Ionization of water |
NH4+ + H20 ⇄ NH3 + H3O+ |
17. Write the equilibrium expressions for each of the above equations except for the second and third reactions.
18. A student tested the electrical conductivity of two acid solutions. One solution was a strong acid and the other a weak acid. Both solutions had the same conductivity. Explain how this could be possible.
19. Describe in terms of hydrolysis how NaCH3COO can be added to potato chips in order to produce the vinegar flavour.
20. Describe what happens to the H+ and the OH- when the pH increases by 2 units.
21. Describe two gases responsible for acid rain. Write equations to show how they react with water. What gas naturally lowers the pH of normal rain?
22. Complete the following reaction using a formula equation, complete ionic quation and net ionic equation. H2C2O4 + NaOH →
23. Write the equilibrium expression for phosphoric acid in water.
24.
|
a) An indicator HInd is red in acid and blue in base. Write the equation for the indicator and explain the colours. |
|
|
b) What is true at the transition point? |
|
|
c)What is the color of this indicator in a solution of AlCl3? |
|
|
d) In the above solution, what is larger [HInd] or [Ind-] ? |
|
|
e) Calculate the Ka for Phenolphthalein. |
|
|
f) What indicator has a Ka of approximately 1.0 X 10-10? |
|
25. Give an example of a monoprotic, diprotic, and triprotic acid. Write an equation for each to show how they ionize in water.
26. Give the approximate pH of the equivalence for each titration. Choose an appropriate indicator.
|
Acid |
Base |
pH of Equivalence Point |
Indicator |
|
HCl |
NaOH |
|
|
|
H2SO4 |
NH3 |
|
|
|
HF |
KOH |
|
|
27. Which of the following will have the lowest pH?
|
HCLO |
HClO2 |
HClO3 |
HClO4 |
28. Pick the formulae that are amphiprotic.
|
H2SO4 |
H20 |
F- |
HCO3- |
|
CO3-2 |
KOH |
H2PO4- |
HPO4-2 |
CALCULATIONS
1. Calculate the quantities required to complete the table. In the first row write the general equations for each quantity. Watch your significant figures.
|
[H+] = |
[OH-] = |
pH = |
pOH = |
|
|
[H+] |
[OH-] |
pH |
pOH |
Acid/base/neutral |
|
5.0 x 10-3 M |
|
|
|
|
|
|
1.3 x 10-5M |
|
|
|
|
|
|
3.1 |
|
|
|
|
|
|
2.508 |
|
|
|
|
|
|
neutral (2sig figs) |
2. What volume of 0.20 M H2SO4 is needed to neutralize 50.00 ml 0.30M NaOH?
3. What mass of NaF is required to prepare 100.0 ml of 0.300 M solution?
4. 35.5 mL of 0.300 M NaOH is required to neutralize 10.0 mL of H2SO4. What is the acid concentration?
5. 100.0 mL of .200 M HCl is mixed with 120.0 mL of 0.200M NaOH. Calculate the pH of the resulting solution.
6. Calculate the Ka for phenolphthalein.
7. The Ksp of AgOH is 6.8 x 10-12. Calculate the pH.
8. The OH- concentration in 0.10M NaCN is 2.7 x 10-6 M. Calculate the Kb from this information only.
9. Calculate the pH for 0.20 M HCl.
10. Calculate the pH for 0.10M Ba(OH)2.
11. Calculate the pH for 0.40 M HCN.
12. Calculate the pH for 0.40 M Na2CO3.
13. What is the pH for 0.30 M NaCl?
14. Calculate the pH of 0.20 M NH3.
15. Calculate the pH of 0.20 M NH4Cl.
16. Show by calculation if H2PO4- is an acid or base (compare the Kb and Ka).
17. Calculate the pH of a saturated solution of Mg(OH)2 if the Ksp is 1.2 X 10-11.
18. A 0.50 M NH3 solution is found to have a OH- concentration of 1.86 x 10-3 M. Using this data only calculate the Kb.
19. A 0.18 M acid HX has a pH of 2.40. What is the Ka?
20. The following data were recorded when 25.00 mL of H2SO4 were titrated with 0.1030 M NaOH. The volumes of NaOH used in three runs were: 46.06 mL, 44.52 mL, 44.54 mL. Calculate the acid concentration.
21. The following data were recorded when 10.00ml of NaOH were titrated with 0.1030M H2SO4. The volumes of H2SO4 used in three runs were: 12.55 mL, 12.55 mL, 12.10 mL. Calculate the base concentration.
Acids Practice Test # 1
1. An equation
representing the reaction of a weak acid with water is
A. HCl
+ H2O ⇄ H3O+ + Cl-
B. NH3 + H2O ⇄ NH4+ + OH-
C. HCO3- H2O ⇄ H2CO3 + OH-
D. HCOOH
+ H2O ⇄ H3O+ +
HCOO-
2. The
equilibrium expression for the ion product constant of water is
A. Kw
= [H3O+][OH-]
[H2O]
B. Kw
= [H3O+]2[O2]
C. Kw
= [H3O+][OH-]
D. Kw
= [H3O+]2[O2-]
3. Consider the
following graph for the titration of 0.1 M NH3 with 1.0 M HCl.
pH Volume HCl added 14 7 0 I II III IV
![]()
![]()

A buffer solution is present at
point
A. I
B. II
C. III
D. IV
4. Consider the
following equilibrium system for an indicator: HInd + H2O ⇄ H3O+ +
Ind-
Which two species must be of two
different colours in order to be used as an indicator?
A. HInd
and H2O
B. HInd
and Ind-
C. H3O+ and Ind-
D. Hind
and H3O+
5. Which of the
following indicators is yellow at pH 10.0?
A. methyl red
B. phenol red
C. thymol blue
D. methyl violet
6. A sample containing 1.20 x 10-2
mole HCl is completely neutralized by 100.0 mL of Sr(OH)2. What is
the [Sr(OH)2]?
A. 6.00
x 10-3 M
B. 6.00
x 10-2 M
C. 1.20
x 10-1 M
D. 2.4 x
10-1 M
7. Which of the
following titrations will have the highest pH at the equivalence point?
A. HBr with NH3
B. HNO2 with KOH
C. HCl with Na2CO3
D. HNO3 with NaOH
8. An Arrhenius acid is defined as a
chemical species that
A. is a proton donor.
B. is a proton acceptor.
C. produces hydrogen ions in solution.
D. produces hydroxide ions in solution.
9. Consider the following acid-base equilibrium system:
HC2O4- +
H2BO3- ⇄
H3BO3 + C2O42-
Identify the Bronsted-Lowry bases
in this equilibrium.
A. H2BO3- and H3BO3
B. HC2O4- and H3BO3
C. HC2O4- and C2O42-
D. H2BO3- and C2O42-
10. The equation
representing the predominant reaction between NaCH3COO with water is
A. CH3COO- + H2O ⇄ CH3COOH + OH-
B. CH3COO- + H2O ⇄ H2O + CH2COO2-
C. CH3COOH + H2O ⇄ H3O+ + CH3COO-
D. CH3COOH + H2O ⇄ CH3COOH2+ + OH-
11. Which of the
following solutions will have the lowest electrical conductivity?
A. 0.10 M HF
B. 0.10 M NaF
C. 0.10 M H2SO3
D. 0.10 M NaHSO3
12. Which of the
following is the strongest Bronsted-Lowry base?
A. NH3
B. CO32-
C. HSO3-
D. H2BO3-
13. A 1.0 x 10-4
M solution has a pH of 10.00. The solute is a
A. weak acid
B. weak base
C. strong acid]
D. strong base
14. The
ionization of water at room temperature is represented by
A. H2O ⇄ 2H+ + O2-
B. 2H2O ⇄
2H2 + O2
C. 2H2O ⇄ H2
+ 2OH-
D. 2H2O ⇄ H3O+ + OH-
15. Addition of
HCl to water causes
A. both [H3O+] and
[OH-] to increase
B. both [H3O+] and
[OH-] to decrease
C. [H3O+] to increase
and [OH-] to decrease
D. [H3O+] to decrease
and [OH-] to increase
16. Consider the
following:
I. H2SO4
II. HSO4-
III. SO42-
Which of the above is/are present
in a reagent bottle labeled 1.0 M H2SO4?
A. I only
B. I and II only
C. II and III only
D. I, II, and III
17. The pH of
0.10 M KOH solution is
A. 0.10
B. 1.00
C. 13.00
D. 14.10
18. An indicator changes colour in the pH
range 9.0 to 11.0. What is the value of the Ka for the indicator?
A. 1
x 10-13
B. 1
x 10-10
C. 1
x 10-7
D. 1
x 10-1
19. Which of the
following are amphiprotic in aqueous solution?
I. HBr
II. H2O
III. HCO3-
IV. H2C6H5O7-
A I and II only
B. II and IV only
C. II, III, and IV only
D. I, II, III, and IV
20. Which of the
following always applies at the transition point for the indicator Hind?
A. [Ind-] =
[OH-]
B. [HInd]
= [Ind-]
C. [Ind-] = [H3O+]
D. [HInd]
= [H3O+]
21. Calculate the [H3O+]
of a solution prepared by adding 10.0 mL of 2.0 M HCl to 10.0 mL of 1.0 M NaOH.
A. 0.20 M
B. 0.50 M
C. 1.0 M
D. 2.0 M
22. Both acidic
and basic solutions
A. taste sour
B. feel slippery
C. conduct electricity
D. turn blue litmus red
23. The conjugate
acid of HPO42- is
A. PO43-
B. H2PO4-
C. H2PO42-
D. H2PO43-
24. What is the
value of the Kw at 25 oC?
A., 1.0
x 10-14
B. 1.0
x 10-7
C. 7
D. 14
25. Consider the
following equilibrium: 2H2O(l) ⇄ H3O+(aq) +
OH-(aq)
A small amount of Fe(H2O)63+
is added to water and equilibrium is re-established. Which of the
following represents the changes in ion concentrations?
[H3O+] [OH-]
A. increases increases
B. increases decreases
C. decreases decreases
D. decreases increases
26. Consider the
following equilibrium for an indicator: HInd
+ H2O ⇄ H3O+ +
Ind-
In a solution of pH of 6.8, the
colour of bromthymol blue is
A. blue because [HInd] =
[Ind-]
B. green because [HInd] = [Ind-]
C. green because [HInd] <
[Ind-]
D. yellow because [HInd] >
[Ind-]
27. The indicator
with Ka = 4 x 10-8 is
A. neutral red
B. methyl red
C. indigo carmine
D. phenolphthalein
28. In a titration a 25.00 mL sample of Sr(OH)2
is completely neutralized by 28.60 mL of 0.100 M HCl. The concentration of the
Sr(OH)2 is
A. 1.43
x 10-3 M
B. 2.86
x 10-3 M
C. 5.72
x 10-2 M
D. 1.14
x 10-1 M
29. A student mixes 15.0 mL of 0.100 M NaOH
with 10.0 mL of 0.200 M HCl. The resulting solution is
A. basic
B. acidic
C. neutral
D. amphiprotic
30. Which of the
following salts will dissolve in water to produce a neutral solution?
A. LiF
B. CrCl3
C. KNO3
D. NH4Cl
31. What is the
value of the Kb for HC6H5O72-?
A. 5.9
x 10-10
B. 2.4
x 10-8
C. 4.1
x 10-7
D. 1.7
x 10-5
32. The pOH of
0.015 M HCl solution is
A. 0.97
B. 1.80
C. 12.18
D. 13.03
33. Which of the
following will produce an acidic solution?
A. NaCl
B. NH4NO3
C. Ca(NO3)2
D. Ba(NO3)2
34. Which of the
following salts will dissolve in water to produce an acid solution?
A. LiF
B. CrCl3
C. KNO3
D. NaCl
35. Which of the
following salts will dissolve in water to produce a basic solution?
A. LiF
B. CrCl3
C. KNO3
D. NH4Cl
36. A student mixes 400 mL of 0.100 M NaOH
with 100 mL of 0.200 M H2SO4. The resulting solution has
a pH of
A. 14.000
B. 0.000
C. 13.800
D. 7.000
37. A student mixes 500 mL of 0.400 M NaOH
with 500 mL of 0.100 M H2SO4. The resulting solution has
a pH of
A. 14.000
B. 0.000
C. 13.000
D. 7.000
38. The strongest
acid in water is
A. HClO4
B. HI
C. HF
D. H3O+
39. The formula
that has the highest pH in water is
A. HF
B. H2CO3
C. H2C2O4
D. HCN
39. The formula
that has the highest pH in water is
A. NaF
B. NaCl
C. H2C2O4
D. NaCN
Subjective
1. A chemist
prepares a solution by dissolving the salt NaCN in water.
a)
Write the equation for the dissociation reaction that occurs.
b) Write the equation for the
hydrolysis reaction that occurs.
c) Calculate the value of the
equilibrium constant for the hydrolysis
2. A 3.50
x 10-3 M sample of
unknown acid, HA has a pH of 2.90. Calculate the value of the Ka and identify
this acid.
3. Calculate
the mass of NaOH needed to prepare 2.0 L of a solution with a pH of 12.00.
4. A 1.00 M OCl-
solution has an [OH-] of 5.75 x 10-4 M. Calculate the Kb for OCl-.
5. Calculate the pH of a solution prepared
by adding 15.0 mL of 0.500 M H2SO4 to 35.0 mL of 0.750 M
NaOH.
6. Determine
the pH of a 0.10 M solution of hydrogen cyanide.
7. Determine
the pH of 0.100 M NH3.
8. Determine
the pH of a saturated solution of Mg(OH)2.
Acids Practice Test # 2
1. What colour would 1.0 M HCl be in an indicator mixture consisting of phenol red and thymolphthalein?
A red
B blue
C yellow
D colourless
2. During a titration, what volume of 0.500 M KOH is necessary to completely neutralize 10.0 mL of 2.00 M CH3COOH?
A 10.0 mL
B 20.0 mL
C 25.0 mL
D 40.0 mL
3. Which indicator has a Ka = 1.0 x 10-6?
A neutral red
B thymol blue
C thymolpthalein
D chlorophenol red
4. Acid is added to a buffer solution. When equilibrium is reestablished the buffering effect has resulted in [H3O+]
A increasing slightly
B decreasing slightly
C increasing considerably
D decreasing considerably
5. A buffer solution will form when 0.10 M NaF is mixed with an equal volume of
A 0.10 M HF
B 0.10 M HCl
C 0.10 M NaCl
D 0.10 M NaOH
6. Which of the following statements applies to 1.0 M NH3(aq) but not to 1.0 M NaOH(aq)?
A partially ionizes
B neutralizes an acid
C has a pH greater than 7
D turns bromocresol green from yellow to blue
7. In which of the following are the reactants favoured?
A HNO2 + CN- ⇄ NO2- + HCN
B H2S + HCO3- ⇄ HS- + H2CO3
C H3PO4 + NH3 ⇄ H2PO4- + NH4+
D CH3COOH + PO43- ⇄ CH3COO- + HPO42-
8. What is the pOH of a solution prepared by adding 0.50 moles of NaOH to prepare 0.50 L of solution?
A 0.00
B 0.30
C 14.00
D 13.70
9. What is the [H3O+] in a solution with a pH = 5.20?
A 1.4 x 10-14
B 1.6
x 10-9
C 6.3
x 10-6
D 7.1 x 10-1
10. Consider the following equilibrium: 2H2O(l) + energy ⇄ H3O+(aq) + OH-(aq)
What will cause the pH to increase and the Kw to decrease?
A adding a strong acid
B adding a strong base
C increasing the temperature
D decreasing the temperature
11. The complete neutralization of 15.0 mL of KOH requires 0.0250 moles H2SO4. The [KOH] was
A 1.50 M
B 1.67 M
C 3.33 M
D 6.67 M
12. What is the [H3O+] at the equivalence point for the titration between HBr and KOH?
A 1.0 x 10-9 M
B 1.0 x 10-7 M
C 1.0 x 10-5 M
D 0.0 M
13. Which of the following would form a buffer solution when equal moles are mixed together?
A HCl and NaCl
B HCN and NaCN
C KNO3 and KOH
D Na2SO4 and NaOH
14. Which of the following acids has the weakest conjugate base?
A HIO3
B HNO2
C H3PO4
D CH3COOH
15. When 10.0 ml of 0.10 M HCl is added to 10.0 mL of water, the concentration of H3O+ in the final solution is
A 0.010 M
B 0.050 M
C 0.10 M
D 0.20 M
16. The conjugate base of an acid is produced by
A adding a proton to the acid
B adding an electron to the acid
C removing a proton from the acid
D removing an electron from the acid
17. Which of the following represents the predominant reaction between HCO3- and water?
A 2HCO3- ⇄ H2O + 2CO2
B HCO3- + H2O ⇄ H2CO3 + OH-
C HCO3- + H2O ⇄ H3O+ + CO32-
D 2HCO3- + H2O ⇄ H3O+ + CO32- + OH- + CO2
18. Water acts as an acid when it reacts with
I CN-
II NH3
III HClO4
IV CH3COO-
A I and IV only
B II and III only
C I, II, and IV
D II, III, and IV
19. In a solution of 0.10 M H2SO4, the ions present in order of decreasing concentration are
A [H3O+] > [HSO4-] > [SO42-] > [OH-]
B [H3O+] > [SO42-] > [HSO4-] > [OH-]
C [OH-] > [SO42-] > [HSO4-] > [H3O+]
D [SO42-] > [HSO4-] > [OH-] > [H3O+]
20. Which of the following will dissolve in water to produce an acidic solution?
A CO2
B CaO
C MgO
D Na2O
21. Which of the following solutions will have a pH = 1.00?
I 0.10 M HCl
II 0.10 M HNO2
III 0.10 M NaOH
A I only
B II only
C I and II only
D I, II, and III
22. Ka for the acid H2AsO4- is 5.6 x 10-8. What is the value of the Kb for HAsO42-?
A 5.6 x 10-22
B 3.2 x 10-14
C 1.8 x 10-7
B 2.4 x 10-4
23. In a titration, which of the following has a pH = 7.00 at the equivalence point?
A NH3 and HNO3
B KOH and HCl
C NaF and HCl
D Ca(OH)2 and CH3COOH
24. Which of the following salts dissolves to produce a basic solution?
A KCl
B NH4Br
C Fe(NO3)3
D LiCH3COO
25. Calculate the pH in a 0.200 M solution of Sr(OH)2.
A 1.40
B 1.70
C 13.30
D 13.60
26. Which of the following solutions has a pH less than 7.00?
A NaCl
B LiOH
C NH4NO3
D KCH3COO
27. Which of the following will form a basic aqueous solution?
A HSO3-
B HSO4-
C HPO42-
D HC2O4-
28. What is the approximate Ka value for the indicator chlorophenol red?
A 1 x 10-14
B 1 x 10-8
C 1 x 10-6
D 1 x 10-3
29. What is the approximate pH of the solution formed when 0.040 mol NaOH is added to 2.00 L of 0.020 M HCl?
A 0.00
B 1.40
C 1.70
D 7.00
30. In which one of the following equations are the Bronsted acids and bases all correctly identified?
Acid + Base ⇄ Base + Acid
A H2O2 SO32- ⇄ HO2- HSO3-
B H2O2 SO32- ⇄ HSO3- HO2-
C SO32- H2O2 ⇄ HO2- HSO3-
D SO32- H2O2 ⇄ HSO3- HO2-
31. Which of the following titrations will always have an equivalence point at a
pH > 7.00?
A weak acid with a weak base
B strong acid with a weak base
C weak acid with a strong base
D strong acid with a strong base
32. A buffer solution may contain equal moles of
A weak acid and strong base
B strong acid and strong base
C weak acid and its conjugate base
D strong acid and its conjugate base
33. A gas which is produced by burning coal and also contributes to the formation of acid rain is
A H2
B O3
C SO2
D C3H8
34. Which of the following 1.0 M salt solutions is acidic?
A BaS
B NH4Cl
C Ca(NO3)2
D NaCH3COO
35. Which of the following statements applies to 1.0 M NH3(aq) but not to 1.0 M NaOH(aq)?
A partially ionizes
B neutralizes and acid
C has a pH greater than 7
D turns bromcresol green from yellow to blue
36. When the indicator thymol blue is added to 0.10 M solution of an unknown acid, the solution is red. The acid could be
A HF
B H2S
C HCN
D HNO3
Subjective
1. Calculate the pH of the solution prepared by mixing 15.0 mL of 0.50 M HCl with 35.0 mL 0.50 M NaOH.
2. Calculate the [OH-] in 0.50 M NH3(aq).
3. A titration was performed by adding 0.175 M H2C2O4 to a 25.00 mL sample of NaOH.
The following data was collected.
Trial 1 Trial 2 Trial 3
Final volume of H2C2O4 from burette (mL) 23.00 39.05 20.95
Initial volume of H2C2O4 from burette (mL) 4.85 23.00 5.00
Calculate the [NaOH]
4. A 250.0 mL sample of HCl with a pH of 2.000 is completely neutralized with 0.200 M NaOH. What volume of NaOH is required to reach the stoichiometric point.
5. If the HCl were titrated with 0.200 M NH3(aq) instead of 0.200 M NaOH, how would the volume of base required to reach the equivalence point compare with the volume calculated in the last question? Explain your answer.
6. Consider the following salt ammonium acetate, NH4CH3COO.
a) Write the equation for the dissociation of NH4CH3COO.
b) Write the equations for the hydrolysis reactions that occur.
c) Explain why a solution of NH4CH3COO has a pH =7.00. Support your answer with a calculation.
7. Consider the following equilibrium: energy + 2H2O ⇄ H3O+ + OH-
a) Explain how pure water can have a pH = 7.30.
b) Calculate the value of the Kw for the sample of water with a pH = 7.30.