Back to W. J. Mouat Chem 12 Home Page
1) Which most readily gains electrons?
|
Cu |
Cu+2 |
Fe+2 |
Zn+2 |
Au+3 |
2) Which most readily loses electrons?
|
Hg(l) |
Cu+2 |
Sn+4 |
Ba |
Al |
Calculate the cell potentials or voltages (E0) Indicatespontaneity.
3. Cl2 + 2Br- -----> 2Cl- +Br2
4. 2MnO4- + 5Pb + 16H+ ----->2Mn+2 + 8H2O + 5Pb+2
5. Will AgNO3 react with Zn? Write a balanced redoxreaction and calculate Eo
6. What would happen if you used an iron spoon to stir a soulutionof Al2(SO4)3(aq) ? Write a balancedredox reaction and calculate Eo.
7) What are the differences between an electrochemical cell and anelectrolytic cell?
|
electrochemical cell |
electrolytic cell |
|
|
|
|
|
|
|
|
|
|
|
|
8) What are the similarities between an electrochemical cell andan electrolytic cell?
|
electrochemical cell or electrolytic cell |
|
|
|
|
|
|
|
|
9) State how you would determine each of the following in anelectrochemical or electrolytic cell.
|
|
Electrochemical Cell |
Electrolytic Cell |
|
The site of reduction |
|
|
|
The site of oxidation |
|
|
|
The +ve electrode |
|
|
|
The -ve electrode |
|
|
|
The anions migrate to the |
|
|
|
The cations migrate to the |
|
|
|
The electrode that gains mass |
|
|
|
The electrode that loses mass |
|
|
|
The electrons flow from |
|
|
10.a) Draw an operating electrochemical cellusing
an Al half-cell and a Mg half-cell. Label the parts of theelectrochemical cell
including the anode or cathode, and all reagentsand materials used. Write the
reactions and determine theE0
11. (a) Write the half reaction that occursat each electrode during the electrolysis of aqueous 1 M NaI.
Anode :
Cathode :
(b) What is the minimum required voltage forthis process?
12. (a) Write the half reaction that occursat each electrode during the electrolysis of molton NaI.
Anode :
Cathode :
(b) What is the minimum required voltage forthis process?
13. Aluminum is produced industrially from aluminum oxide,Al2O3. Demonstrate your understanding of thisprocess by
(i) describing how the process is carried out,
(ii) writing equations of the reactions involved in the process,and
(iii) describing how the problem of the high melting point ofAl2O3 is overcome.
14. Consider the following redoxdata:
3V
+2Ga3+
3V
Based on these observations, a studentconcludes
that Ga
15. Balance the equation for the followinghalf
reaction occuring in acid solution:
16. Balance the following redox reactionoccuring
in basic solution:
17. 250ml .200M MnO4- reacts with excessSO3-2. How many grams of MnO2 areproduced?
2MnO4- + 3SO3-2 +H2O -----> 2MnO2 +3SO4-2 + 2OH-
18. Determine the oxidation number for each bold atom.
|
MnO2 |
IO3- |
Cr2O7-2 |
C2O4-2 |
Al(NO3)3 |
NH4Cl |
NaH |
|
|
|
|
|
|
|
|
|
HOOH |
NO3- |
H3PO4 |
Na2C2O4 |
I2 |
N2O3 |
Pt(H2O)4+2 |
|
|
|
|
|
|
|
|
19. 250ml of .500M MnO4- are required totitrate a 100ml sample of SO3-2. Calculate the[SO3-2]
2MnO4- + 3SO3-2 +H2O -----> 2MnO2 + 3SO4-2+ 2OH-
20. How is the breathalyzer reaction used to determine BAC? Writethe reaction and describe how it works.
21. 2H+ + Mg-----> Mg+2 +H2
Determine the Oxidizing agent__________ and the Reducingagent_________
22. Choose a suitable redox reactant to oxidize Cl- toClO4- in a redox titration.
23. Describe as an electrochemical or electrolytic cell:
|
a) Fuel cell |
|
|
b) Charging a car battery |
|
|
c) Discharging a car battery |
|
|
d) Ni plating |
|
|
e) Industrial Al production |
|
|
f) Cl2 production |
|
|
g) Electrowinning |
|
24) Which of the reactants is gaining electrons? Which of thereactants is the oxidizing agent?
Br2 + SO2+Na2SO4 +H2O -------->2H2SO4 + 2NaBr
25) A student studied the following reactions and she recorded:
Pd+2 + Cu -------> Pd + Cu+2 spontaneous
Pd+2 + Au -------> no reaction
Pd+2 + Hg -------> no reaction
Au+3 + Hg -------> Au + Hg+2 spontaneous
List the oxidizing agents from strongest to weakest. List thereducing agents from strongest to weakest. Predict if the reactionwill occur.
Au+3 + Cu ------------>
26) Match each type of electrolytic cell with the example cell.
|
Electrowinning |
A silver anode oxidizes & Ag reduces on a Cu cathode |
|
Electroplating |
Pure Pb is reduced at the cathode while impure Pb oxidizes at the anode |
|
Electrorefining |
Pure Al is reduced at the cathode from molten bauxite (Al2O3). |
27. List the anode, cathode, anode reaction , cathode reaction,and
electrolyte for each commercial electrochemical cell.
|
Cell |
anode |
anode reaction |
cathode |
cathode reaction |
electrolyte |
|
Leclanche or Common Dry Cell |
|
|
|
|
|
|
Alkaline Cell |
|
|
|
|
|
|
Lead Storage or Car Battery |
|
|
|
|
|
|
Fuel Cell |
|
|
|
|
|
28. Which of the above cells requires continuous input ofO2 and H2 and is produced by BallardIndustries.
29. List the anode, cathode, anode reaction , cathode reaction,and electrolyte for each commercial electrolytic cell.
|
Cell |
anode |
anode reaction |
cathode |
cathode reaction |
electrolyte |
|
Electrolysis of Molten Al2O3 |
|
|
|
|
|
|
Electrolysis of Aqueous NaCl |
|
|
|
|
|
|
Silver-plating a Cu plating |
|
|
|
|
|
|
Electrorefining pure Pb from impure Pb |
|
|
|
|
|
30. Describe each term:
|
salt bridge |
|
|
electrolyte |
|
|
anode |
|
|
cathode |
|
|
spontaneous |
|
|
electronegativity |
|
|
cation |
|
|
anion |
|
|
electrochemical cell |
|
|
electrolytic cell |
|
|
oxidation number |
|
|
electrolysis |
|
|
oxidation |
|
|
reduction |
|
|
oxidizing agent |
|
|
reducing agent |
|
|
electrode |
|
|
corrosion |
|
|
electrowinning |
|
|
electrorefining |
|
|
overpotential effect |
|
|
fuel cell |
|
31. Define corrosion of a metal, and illustrate yourdefinition with reference to an example, using appropriate equations.Give TWO methods by which corrosion can be prevented and describe howeach method works. The two methods must involve different chemicalprinciples.
32. Explain why you would choose Zn or Cu to cathodically protectiron?
33. A+2 does not react with B, while C+2 reacts with B. Rank theoxidizing agents in decreasing order of strength. Rank the reducingagents in decreasing order of strength. Will A+2 react with C?
34. Write half reactions for each using the reduction table andlist the half cell potential.
|
|
Half Reaction |
Eo |
|
oxidation of water |
|
|
|
oxidation of water in acid |
|
|
|
reduction of water |
|
|
|
reduction of water in alkaline |
|
|
|
oxidation of H2 in water |
|
|
|
oxidation of H2 in acid |
|
|
|
oxidation of H2 in base |
|
|
|
reduction of Cr2O7-2 in acid |
|
|
|
reduction of HBr |
|
|
35.Completely analyze the following electrochemical cell.
|
|
|
|
The cathode reaction is: |
|
|
The electrons flow from ___ to ___ |
|
|
The ions that migrate to the Zn electrode are: |
|
|
The ions that migrate to the Cu electrode are: |
|
|
The intial voltage of this cell is: |
|
|
The voltage of this cell once equilibrium is reached is: |
|
|
Describe the change in [Cu+2] in the Cu half cell |
|
|
Describe the change in [NO3-1] in the Zn half cell |
|
36. Completely analyze the following electrochemical cell.
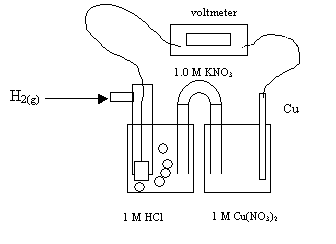
|
The anode reaction is: |
|
|
The cathode reaction is: |
|
|
The electrons flow from ___ to ___ |
|
|
The ions that migrate to the Pt electrode are: |
|
|
The ions that migrate to the Cu electrode are: |
|
|
The intial voltage of this cell is: |
|
|
The voltage of this cell once equilibrium is reached is: |
|
|
Describe the change in [Cu+2] in the Cu half cell |
|
|
Describe the change in [NO3-1] in the H+/H2 half cell |
|
37. Completely analyze the following electrolytic cell.
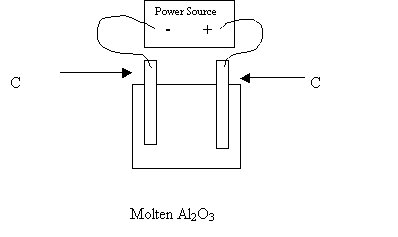
|
Anode Reaction |
|
|
Cathode Reaction |
|
|
Chemicals produced at the anode |
|
|
Chemicals produced at the cathode |
|
|
The electrons flow from __to __ |
|
|
The chemical used to lower the mp is: |
|
|
Which electrode is the anode ? |
|
38. Completely analyze the following electrolytic cell. Note that the electrodes are not inert and because of that, the anode might oxidize.
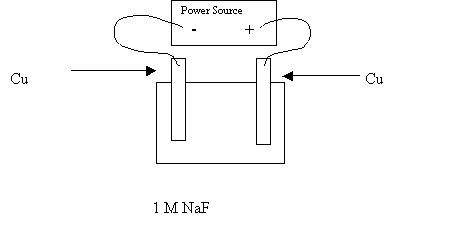
|
Anode Reaction |
|
|
Cathode Reaction |
|
|
Chemicals produced at the anode |
|
|
Chemicals produced at the cathode |
|
|
The electrons flow from |
|
|
The MTV |
|
|
Which electrode is the anode ? |
|