Acids Quiz #10 Review Answers
1. How many moles of Mg(OH)2 are required to neutralize 30.00 mL of 0.150 M
HCl?
A. 2.25
x 10-3 mol
B. 4.50 x 10-3 mol
C. 5.00 x 10-3 mol
D. 9.00 x 10-3 mol
2. The approximate Ka for the indicator phenolphthalein is
A. 6 x 10-19
B. 8
x 10-10
C. 6 x 10-8
D. 2 x 10-2
3. A new indicator, “B.C. Blue (HInd),” is red in bases and blue in acids. Describe
the shift in equilibrium and the resulting color change if 1.0 M HIO3 is added to a
neutral, purple solution of this indicator:
HInd + H2O ⇄
H3O+ +
A. Equilibrium shifts left, and colour becomes red
B Equilibrium
shifts left, and colour becomes blue
C. Equilibrium shifts right, and colour becomes red
D. Equilibrium shifts right, and colour becomes blue
4. Which one of the following combinations would act as an acid buffer?
A. HCl and NaOH
B. KOH and KBr
C. NH3 and NH4Cl
D. CH3COOH
and NaCH3COO
5. What is the pH at the transition point of an indicator if its Ka is 7.9 x 10-3?
A. 0.98
B. 2.10
C. 7.00
D. 11.90
6. Which of the following curves best represents the titration of sodium hydroxide
with hydrochloric acid?
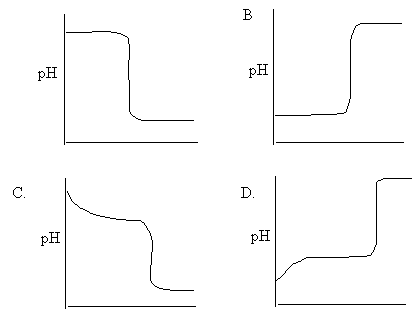
A.
7. A student prepares a buffer by placing ammonium chloride in a solution of
ammonia. Equilibrium is established according to the equation:
NH3
+ H2O ⇄
NH4+ +
When a small amount of base is added to the buffer, the base reacts with
A. NH3 and the pH decreases
B. NH4+ and the pH decreases
C. NH3 to keep the pH relatively constant
D. NH4+
to keep the pH relatively constant
8. At the equivalence point in a titration involving 1.0 M solutions, which of the
following combinations would have the lowest conductivity?
A. Nitric acid and barium hydroxide
B. Acetic acid and sodium hydroxide
C. Sulphuric
acid and barium hydroxide
D. Hydrochloric acid and sodium hydroxide
9. An indicator HInd produces a yellow colour in 0.1 M HCl solution and a red
colour in 0.1 M HCN solution. Therefore, the following equilibrium:
HCN +
A. Products are favored and the stronger acid is HInd
B. Products are favored and the stronger acid is HCN
C. Reactants
are favored and the stronger acid is HInd
D. Reactants are favored and the stronger acid is HCN
10. The indicator methyl red is red in a solution of NaH2PO4. Which of the following
equations is consistent with this observation?
A. H2PO4-
+ H2O ⇄ HPO42- + H3O+
B. H2PO4-
+ H2O ⇄
H3PO4 +
C. HPO42- + H2O ⇄ PO43- + H3O+
D. HPO42- + H2O
⇄ H2PO4-
+
11. Consider the following acid-base indicator equilibrium:
HInd(aq) + H2O(l) ⇄ H3O+(aq) ⇄
Which of the following statements describes the conditions that exist in an
indicator equilibrium system at its transition point?
A. [HInd] = [
B. [
C. [HInd] = [H3O+]
D. [H3O+] = [H2O]
12. Which of the following titrations would have an equivalence point less that pH 7?
A. NH3 and HCl
B. NaOH and HNO3
C. Ba(OH)2 and H2SO4
D. KOH and CH3COOH
13. Oxalic acid dihydrate is a pure, stable, crystalline substance. Which of the following describes
one of its uses in acid-base titrations?
A. buffer
B. primary standard
C. indicator
D. acid anhydride
14. What is the complete ionic equation that describes the reaction of HCl(aq) with Pb(OH)2(s)?
A. H+(aq)
+
B. 2HCl(aq) + Pb(OH)2(s) ® PbCl2(s) + 2H2O(l)
C. 2H+(aq) + 2Cl-(aq) + Pb(OH)2(s) ® PbCl2(s) + 2H2O (l)
D. 2H+(aq) + 2Cl-(aq) + Pb2+(aq) + 2OH- ®Pb2+(aq) + 2Cl-(aq) + 2H2O (l)
15. What is the formula equation that describes the reaction of HCl(aq) with Pb(OH)2(s)?
A. H+(aq)
+
B. 2HCl(aq) + Pb(OH)2(s) ® PbCl2(s) + 2H2O(l)
C. 2H+(aq) + 2Cl-(aq) + Pb(OH)2(s) ® PbCl2(s) + 2H2O (l)
D. 2H+(aq) + 2Cl-(aq) + Pb2+(aq) + 2OH- ®Pb2+(aq) + 2Cl-(aq) + 2H2O (l)
16. What term is used to describe the point at which a chemical indicator changes
colour?
A. titration point
B. transition point
C. equivalence point
D. neutralization point
17. What term is used to describe the point in a titration where the acid has
completely reacted with the base.
A. titration point
B. transition point
C. equivalence point
D. neutralization point
18. Which of the following is a piece of equipment typically used in acid-base
titrations?
A. burette
B. cuvette
C. litmus paper
D. graduated cylinder
19. Identify an environmental problem associated with acid rain.
A. increasing the pH of lakes
B. the green house effect
C. chemical decomposition of rainwater
D. metals leeching from river rocks accumulating in lakes
20. A buffer solution is prepared using sufficient amounts of H2S and NaHS. What
limits this buffer’s effectiveness when NaOH is added?
A. [H2S]
B. [HS-]
C. [
D. [H3O+]
21. Which of the following is not a good use for an acid-base titration curve?
A. to determine the concentration of the acid
B. to select a suitable indicator for the titration
C. to determine whether the acid is strong or weak
D. to select a suitable primary standard for the titration
22. A substance which completely produces hydroxide ions in solution is a definition
of which of the following?
A. a strong Arrhenius acid
B. a strong Arrhenius base
C. a weak Arrhenius base
D. a strong Brønsted-Lowry base
23. When a strong acid is titrated with a strong base, what will the pH value be at the
equivalence point?
A. 0.0
B. 5.0
C. 7.0
D. 9.0
24. At a certain point in a strong acid-strong base titration, the moles of H+ are equal
to the moles of
A. the end point
B. the titration point
C. the transition point
D. the equivalence point
25. A 25.0 mL sample of H2SO4(aq) is titrated with 15.5 mL of 0.50 M NaOH(aq).
What is the concentration of the H2SO4(aq) ?
A. 0.078 M
B. 0.16 M
C. 0.31 M
D. 0.62 M
26. When a weak acid is titrated with a strong base, what will the pH value be at the
equivalence point?
A. 0.0
B. 6.7
C. 7.0
D. 8.8
27. A substance which completely produces accepts a proton from an acid is a
definition of which of the following?
A. a strong Arrhenius acid
B. a strong Arrhenius base
C. a weak Arrhenius base
D. a strong Brønsted-Lowry base
28. Which of the following is a piece of equipment typically used in acid-base
titrations?
A. pipette
B. test tube
C. litmus paper
D. graduated cylinder
29. Water has the greatest tendency to act as an acid with which of the following?
A. NO3-
B. NO2-
C. H2PO4-
D. CH3COO-
30. Which net ionic equation best describes the reaction between NaOH and H2S?
A. H+(aq)
+
B. H2S(aq) + 2OH- ® 2H2O(l) + S2-(aq)
C. H2S(aq) + 2NaOH ® 2H2O(l) + Na2S(aq)
D. 2H+(aq)
+ S2-(aq) + 2NaOH(aq) ® 2H2O(l) +
2Na+(aq) +
S2-(aq)