1. An equation representing the reaction of a weak acid with water is
A. HCl + H2O ⇄ H3O+ + Cl-
B. NH3 + H2O ⇄ NH4+ + OH-
C. HCO3- H2O ⇄ H2CO3 + OH-
D. HCOOH + H2O ⇄ H3O+ +
HCOO-
2. The equilibrium expression for the ion product constant of water is
A. Kw = [H3O+][OH-]
[H2O]
B. Kw = [H3O+]2[O2]
C. Kw = [H3O+][OH-]
D. Kw = [H3O+]2[O2-]
3. Consider the following graph for the titration of 0.1 M NH3 with 1.0 M HCl.
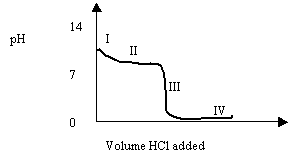
A buffer solution is present at point
A. 5
B. 7
C. 9
D. 0
4. Consider the following equilibrium system for an indicator: HInd + H2O ⇄ H3O+ + Ind-
Which two species must be of two different colours in order to be used as an indicator?
A. HInd and H2O
B. HInd and Ind-
C. H3O+ and Ind-
D. Hind and H3O+
5. Which of the following indicators is yellow at pH 10.0?
A. methyl
red
B. phenol red
C. thymol blue
D. methyl violet
6. A sample containing 1.20 x 10-2 mole HCl is completely neutralized by 100.0 mL of Sr(OH)2. What is the [Sr(OH)2]?
A. 6.00 x 10-3 M
B. 6.00 x 10-2
M
C. 1.20 x 10-1 M
D. 2.4 x 10-1 M
7. Which of the following titrations will have the highest pH at the equivalence point?
A. HBr with NH3
B. HNO2
with KOH
C. HCl with Na2CO3
D. HNO3 with NaOH
8. An Arrhenius acid is defined as a chemical species that
A. is a proton donor.
B. is a proton acceptor.
C. produces
hydrogen ions in solution.
D. produces hydroxide ions in solution.
9. Consider the following acid-base equilibrium system: HC2O4- + H2BO3- ⇄ H3BO3 + C2O42-
Identify the Bronsted-Lowry bases in this equilibrium.
A. H2BO3- and H3BO3
B. HC2O4- and H3BO3
C. HC2O4- and C2O42-
D. H2BO3- and C2O42-
10. The equation representing the predominant reaction between NaCH3COO with water is
A. CH3COO- + H2O ⇄ CH3COOH + OH-
B. CH3COO- + H2O ⇄ H2O + CH2COO2-
C. CH3COOH + H2O ⇄ H3O+ + CH3COO-
D. CH3COOH + H2O ⇄ CH3COOH2+ + OH-
11. Which of the following solutions will have the lowest electrical conductivity?
A. 0.10
M HF
B. 0.10 M NaF
C. 0.10 M H2SO3
D. 0.10 M NaHSO3
12. Which of the following is the strongest Bronsted-Lowry base?
A. NH3
B. CO32-
C. HSO3-
D. H2BO3-
13. A 1.0 x 10-4 M solution has a pH of 10.00. The solute is a
A. weak acid
B. weak base
C. strong acid]
D. strong
base
14. The ionization of water at room temperature is represented by
A. H2O ⇄ 2H+ + O2-
B. 2H2O ⇄ 2H2 + O2
C. 2H2O ⇄ H2
+ 2OH-
D. 2H2O ⇄ 2H3O+ + OH-
15. Addition of HCl to water causes
A. both [H3O+] and [OH-] to increase
B. both [H3O+] and [OH-] to decrease
C. [H3O+]
to increase and [OH-] to decrease
D. [H3O+] to decrease and [OH-] to increase
16. Consider the following:
I. H2SO4
II. HSO4-
III. SO42-
Which of the above is/are present in a reagent bottle labeled 1.0 M H2SO4?
A. I only
B. I and II only
C. II and
III only
D. I, II, and III
17. The pH of 0.10 M KOH solution is
A. 0.10
B. 1.00
C. 13.00
D. 14.10
18. An indicator changes colour in the pH range 9.0 to 11.0. What is the value of the Ka for the indicator?
A. 1
x 10-13
B. 1 x 10-10
C. 1
x 10-7
D. 1 x 10-1
19. Which of the following are amphiprotic in aqueous solution?
I. HBr
II. H2O
III. HCO3-
IV. H2C6H5O7-
A I and II only
B. II and IV only
C. II,
III, and IV only
D. I, II, III, and IV
20. Which of the following always applies at the transition point for the indicator Hind?
A. [Ind-] = [OH-]
B. [HInd] =
[Ind-]
C. [Ind-] = [H3O+]
D. [HInd] = [H3O+]
21. Calculate the [H3O+] of a solution prepared by adding 10.0 mL of 2.0 M HCl to 10.0 mL of 1.0 M NaOH.
A. 0.20 M
B. 0.50
M
C. 1.0 M
D. 2.0 M
22. Both acidic and basic solutions
A. taste sour
B. feel slippery
C. conduct
electricity
D. turn blue litmus red
23. The conjugate acid of HPO42- is
A. PO43-
B. H2PO4-
C. H2PO42-
D. H2PO43-
24. What is the value of the Kw at 25 oC?
A., 1.0 x 10-14
B. 1.0 x 10-7
C. 7
D. 14
25. Consider the following equilibrium: 2H2O(l) ⇄ H3O+(aq) + OH-(aq)
A small amount of Fe(H2O)63+ is added to water and equilibrium is re-established. Which of the following represents the changes in ion concentrations?
[H3O+] [OH-]
A. increases increases
B. increases decreases
C. decreases decreases
D. decreases increases
26. Consider the following equilibrium for an indicator: HInd + H2O ⇄ H3O+ + Ind-
In a solution of pH of 6.8, the colour of bromthymol blue is
A. blue because [HInd] = [Ind-]
B. green
because [HInd] = [Ind-]
C. green because [HInd] < [Ind-]
D. yellow because [HInd] > [Ind-]
27. The indicator with Ka = 4 x 10-8 is
A. neutral
red
B. methyl red
C. indigo carmine
D. phenolphthalein
28. In a titration a 25.00 mL sample of Sr(OH)2 is completely neutralized by 28.60 mL of 0.100 M HCl. The concentration of the Sr(OH)2 is
A. 1.43 x 10-3 M
B. 2.86 x 10-3 M
C. 5.72 x 10-2
M
D. 1.14 x 10-1 M
29. A student mixes 15.0 mL of 0.100 M NaOH with 10.0 mL of 0.200 M HCl. The resulting solution is
A. basic
B. acidic
C. neutral
D. amphiprotic
30. Which of the following salts will dissolve in water to produce a neutral solution?
A. LiF
B. CrCl3
C. KNO3
D. NH4Cl
31. What is the value of the Kb for HC6H5O72-?
A. 5.9 x 10-10
B. 2.4
x 10-8
C. 4.1 x 10-7
D. 1.7
x 10-5
32. The pOH of 0.015 M HCl solution is
A. 0.97
B. 1.80
C. 12.18
D. 13.03
33. Which of the following will produce an acidic solution?
A. NaCl
B. NH4NO3
C. Ca(NO3)2
D. Ba(NO3)2
34. Which of the following salts will dissolve in water to produce an acid solution?
A. LiF
B. CrCl3
C. KNO3
D. NaCl
35. Which of the following salts will dissolve in water to produce a basic solution?
A. LiF
B. CrCl3
C. KNO3
D. NH4Cl
36. A student mixes 400 mL of 0.100 M NaOH with 100 mL of 0.200 M H2SO4. The resulting solution has a pH of
A. 14.000
B. 0.000
C. 13.800
D. 7.000
37. A student mixes 500 mL of 0.400 M NaOH with 500 mL of 0.100 M H2SO4. The resulting solution has a pH of
A. 14.000
B. 0.000
C. 13.000
D. 7.000
38. The strongest acid in water is
A. HClO4
B. HI
C. HF
D. H3O+
39. The formula that has the highest pH in water is
A. HF
B. H2CO3
C. H2C2O4
D. HCN
39. The formula that has the highest pH in water is
A. NaF
B. NaCl
C. H2C2O4
D. NaCN
1. A chemist prepares a solution by dissolving the salt NaCN in water.
a) Write the equation for the dissociation reaction that occurs.
NaCN → Na+ +
CN-
b) Write the equation for the hydrolysis reaction that occurs.
CN- +
H2O ⇄ HCN +
OH-
c) Calculate the value of the equilibrium constant for the hydrolysis
Kb(CN-) = Kw = 1.0 x 10-14 = 2.0 x 10-5
Ka(HCN) 4.9
x 10-10
2. A 3.50 x 10-3 M sample of unknown acid, HA has a pH of 2.90. Calculate the value of the Ka and identify this acid.
[H+] = 10-pH = 10-2.90 = 0.00126 M
HA ⇄ H+ +
A-
I 0.00350 M 0 0
C 0.00126 M 0.00126
M 0.00126 M
E 0.00224 M 0.00126
M 0.00126 M
Ka = (0.00126)2 = 7.1 x 10-4 Citric acid
0.00224
3. Calculate the mass of NaOH needed to prepare 2.0 L of a solution with a pH of 12.00.
pOH = 14.00 -
12.00 = 2.00
[OH-] =
10-2.00 = 0.010
M
2.0 L x 0.010
mol x 40.0 g = 0.80 g NaOH
L
mole
4. A 1.00 M OCl- solution has an [OH-] of 5.75 x 10-4 M. Calculate the Kb for OCl-.
OCl- + H2O ⇄ HOCl + OH-
I 1.00 M 0 0
C 0.000575 M 0.000575 M 0.000575 M
E 0.9994 M 0.000575 M 0.000575 M
Kb = (0.000575)2 = 3.31 x 10-7
0.9994
5. Calculate the pH of a solution prepared by adding 15.0 mL of 0.500 M H2SO4 to 35.0 mL of 0.750 M NaOH.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
0.0150 L x 0.500
moles = 0.00750 moles 0.0350 L x 0.750
moles = 0.02625 moles
L L
I 0.0075 moles 0.02625
moles
C 0.0075 mole 0.0150
moles
E 0 0.01125
mole
[NaOH] =
0.01125 moles = 0.225 M
0.0500 L
pH = 13.354
6. Determine the pH of a 0.10 M solution of hydrogen cyanide.
HCN ⇄ H+ + CN-
I 0.10 M 0 0
C x x x
E 0.10 - x x x
small Ka approximation x =
0
Ka = x2 = 4.9 x 10-10
0.10
x = 7.0 x 10-6 pH =
5.15
7. Determine the pH of 0.100 M NH3.
NH3 + H2O ⇄ NH4+ + OH-
I 1.00 M 0 0
C x x x
E 1.00 - x x x
small Kb approximation x = 0
Kb(NH3) = Kw = 1.0 x 10-14 = 1.786 x 10-5
Ka(NH4+) 5.6 x 10-10
Kb = x2
= 1.786 x 10-5
0.100
x = 4.22 x 10-4 pH = 11.13
8. Determine the pH of a saturated solution of Mg(OH)2.
Mg(OH)2 ⇄ Mg2+ +
2OH-
x x 2x
4x3 = 5.6 x
10-12
[OH-] = 2x = 2.24 x 10-4 pOH = 3.65 pH = 10.35