1. Consider the following reaction mechanism:
Step1: NO(g) + O3(g) → NO2(g) + O2(g)
Step2: O(g) + NO2(g) → NO(g) + O2(g)
The catalyst is:
A. O2
B. O3
C. NO
D. NO2
2. Consider the following reaction: 2NH3(g) ⇄ N2(g) + 3H2(g)
A flask is initially filled with NH3. As the system approaches equilibrium, the rate of the forward reaction
A. increases as the rate of the reverse reaction decreases
B. decreases as the rate of the reverse
reaction increases
C. increases as the rate of the reverse reaction increases
D. decreases as the rate of the reverse reaction decreases
3. Consider the following reaction:
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l) ΔH = -153 KJ
In this reaction
A. minimum
enthalpy and maximum entropy both favour the products
B. minimum enthalpy and maximum entropy both favour the reactants
C. minimum enthalpy favours products and maximum entropy reactants
D. minimum enthalpy favours reactants and maximum entropy products
4. In all systems at equilibrium, the
A. concentration of reactants is less than the concentration of the products
B. concentration of reactants and the concentration of the products are equal
C. concentration of reactants is greater than the concentration of the products
D. concentration
of reactants and the products are constant
5. Consider the following mechanism: Step 1: N2O(g) → N2(g) + O(g)
Step 2: N2O(g) + O(g) → N2(g) + O2(g)
A reactant in the overall equation is
A. O
B. O2
C. N2
D. N2O
6. Chemical systems tend to move toward positions of
A. minimum enthalpy and maximum entropy.
B. maximum enthalpy and minimum entropy.
C. minimum enthalpy and minimum entropy.
D. maximum enthalpy and maximum entropy.
7. An equilibrium system shifts left when the
A. rate of the forward reaction is equal to the rate of the reverse reaction.
B. rate of the forward reaction is less
than the rate of the reverse reaction.
C. rate of the forward reaction is greater than the rate of the reverse reaction.
D. rate of the forward reaction and the reverse reaction are constant.
8. A 1.00 L flask contains a gaseous equilibrium system. The addition of reactants to this flask results in a
A. shift left and a decrease in the concentration of the products.
B. shift left and a increase in the concentration of the products.
C. shift right and a decrease in the concentration of the products.
D. shift right
and a increase in the concentration of the products.
9. Consider the following equilibrium: CH4(g) + H2O(g) + heat ⇄ CO(g) + 3H2(g)
In which of the following will both stresses shift the equilibrium to the right?
A. a decrease in temperature and a decrease in volume
B. a increase in temperature and a decrease in volume
C. a decrease in temperature and a increase in volume
D. a increase
in temperature and a increase in volume
10. Consider the following equilibrium: 2SO2(g) + O2(g) ⇄ 2SO3(g) ∆H = -198 kJ
There will be no shift in this equilibrium when
A. more O2 is added.
B. a catalyst
is added.
C. the volume is increased.
D. the temperature is increased.
11. Consider the following equilibrium: 2Fe(s) + 3H2O(g) ⇄ Fe2O3(s) +
3H2(g)
The equilibrium expression is
A. Keq = [Fe2O3][H2]3 B. Keq = [Fe2O3][3H2]
[Fe]2[H2O]3 [2Fe][3H2O]
C. Keq = [H2]3 D. Keq = [ H2]3
[H2O]3
12. Consider the following equilibrium: N2O4(g) ⇄ 2NO2(g) Keq = 0.133
At equilibrium, the [N2O4] is equal to
A. 0.133
B.
[NO2]
[NO2] 0.133
C. 0.133 D. [NO2]2
[NO2]2 0.133
13. Which of the following equilibrium systems most favours the products?
A. Cl2(g) ⇄ 2Cl(g) Keq = 6.4 x 10-39
B. Cl2(g) +
2NO(g) ⇄ 2NOCl(g) Keq = 3.7 x 108
C. Cl2(g) + 2NO2(g) ⇄ 2NO2Cl(g) Keq = 1.8
D. 2HCl(g) ⇄ H2(g) + Cl2(g) Keq = 2.0 x 10-7
14. Consider the following equilibrium: 4KO2(s) + 2H2O(g) ⇄ 4KOH(s) + 3O2(g)
The equilibrium expression is
A. Keq = [KOH]4[O2]3 B. Keq = [O2]3
[KO2]2[H2O]2 [ H2O]2
C. Keq = [KO2]4[H2O]2 D. Keq
= [ H2O]2
[KOH]4[O2]3 [O2]3
15. Consider the following equilibrium: N2(g) + O2(g) ⇄ 2NO(g) ∆H = +181 kJ
When the temperature is decreased, the equilibrium:
A. shifts left and the Keq value increases
B. shifts
left and the Keq value decreases
C. shifts right and the Keq value increases
D. shifts right and the Keq value decreases
16. Consider the following equilibrium: CaCO3(s) + 556 kJ ⇄ CaO + CO2(g)
The value of the equilibrium constant will increase when
A. CO2 is added.
B. CO2 is removed.
C. the
temperature is increased.
D. the temperature is decreased.
17. Consider the following equilibrium: C(s) + H2O(g) ⇄ CO(g) + H2(g)
The contents of a 1.00 L container at equilibrium were analyzed and found to contain
0.20 mole C, 0.20 mole H2O, 0.60 mole CO, and 0.60 mole H2, The equilibrium constant is
A. 0.11
B. 0.56
C. 1.8
D. 0.0
18. Consider the following equilibrium: N2O4(g) ⇄ 2NO2(g) Keq = 4.61 x 10-3
A 1.00 L container at equilibrium was analyzed and found to contain 0.0200 mole NO2.
At equilibrium, the concentration of N2O4 is
A. 0.0868 M
B. 0.230 M
C. 4.34 M
D. 11.5 M
19. Consider the following potential energy diagram:
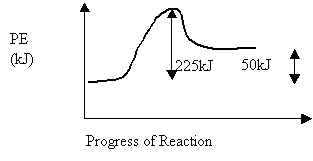
The forward reaction is
A. exothermic and the ∆H = -50 kJ
B. endothermic
and the ∆H = +50 kJ
C. exothermic and the ∆H = -225 kJ
D. endothermic and the ∆H = +225 kJ
20. Consider the following equilibrium: H2O(g) + CO(g) ⇄ H2(g) + CO2(g)
A closed container is initially filled with H2O and CO. As the reaction proceeds to equilibrium the
A. [CO] and [CO2] both increase
B. [CO] and [CO2] both decrease
C. [CO] increases and [CO2] decreases
D. [CO]
decreases and [CO2] increases
21. Consider the equilibrium: H2(g) + I2(g) ⇄ 2HI(g) The pressure of the system is increased by reducing the volume.
When comparing the new equilibrium with the original equilibrium,
A. all concentrations remain constant
B. the
concentrations of all species have
increased
C. reactant concentrations have increased while products decreased
D. reactant concentrations have decreased while products increased
22. Consider the following equilibrium: N2O4(g) ⇄ 2NO2(g) A 1.00 L container is initially filled with
0.200 moles of N2O4. At equilibrium, 0.160 moles NO2 are present. What is the equilibrium concentration of N2O4?
A. 0.040 M
B. 0.080 M
C. 0.120 M
D. 0.160 M
23. Equilibrium is dynamic process because the
A. macroscopic properties are not changing
B. mass of the reactants equals the mass of the products
C. forward
and reverse reactions continue to occur
D. concentrations of reactants and products are constant
24. Consider the following equilibrium: C(s) + 2H2(g) ⇄ CH4(g) The addition of H2 will cause the equilibrium to shift to the
A. left and [CH4] will increase
B. left and [CH4] will decrease
C. right and [CH4] will increase
D. right and [CH4] will decrease
25. Given the following system: 2CrO42-(aq) + 2H+(aq) ⇄ Cr2O72-(aq) + H2O(l)
Which of the following chemicals, when added to the above equilibrium, would result in a decrease in [CrO42-]?
A. NaOH
B. HNO3
C. Na2CrO4
D. Na2Cr2O7
26. Addition of a catalyst to an equilibrium system
A. increases the value of the Keq.
B. increases the yield of the products.
C. has no effect on the rates of the reaction.
D. increases the
rate of formation of both reactants and products.
27. Consider the following reaction: 2B(s) + 3F2(g) ⇄ 2BF3(g) The equilibrium expression is
A. Keq
= [2BF3]
[3F2]
B. Keq
= [F2]3
[BF3]
C. Keq
= [BF3]2
[F2]3
D. Keq = [BF3]2
[B][F2]3
28. Consider the following equilibrium: 2NO(g) ⇄ N2(g)
+ O2(g) Keq = 2.01 x 1030
The value of the equilibrium constant indicates that the
A. [NO]2
< [N2][ O2]
B. [NO]2 > [N2][ O2]
C. [NO] = [N2][ O2]
D. [NO] > [N2][ O2]
29. Consider the equilibrium: H2(g) + I2(g) ⇄ 2HI(g)
At equilibrium the [H2} = 0.020 M, [I2] = 0.020 M, and [HI] = 0.160 M. The value of the equilibrium constant is:
A. 2.5
x 10-3
B. 1.6
x 10-2
C. 6.4 x 101
D. 4.0 x 102
30. Consider the following equilibrium: H2O(g) + Cl2O(g)
⇄ 2HOCl(g) Keq
= 9.0 x 10-2
A 1.0 L flask contains a mixture of 1.8 x 10-1 mole H2O, 4.0 x 10-4 mole Cl2O, and
8.0 x 10-2 mole HOCl. To establish equilibrium, the system will proceed to the
A. left
because the trial Keq > Keq
B. left because the trial Keq < Keq
C. right because the trial Keq > Keq
D. right because the trial Keq < Keq
31. Consider the following equilibrium: SO2(g) + NO2(g) ⇄ SO3(g) + NO + energy
The equilibrium does not shift with a change in
A. volume
B. temperature
C. concentration of products
D. concentration of reactions
32. Consider the following equilibrium : SO2Cl2(g) + energy ⇄ SO2(g) + Cl2(g)
When the temperature is decreased, the equilibrium shifts
A. left and
the [SO2Cl2] increases
B. left and the [SO2Cl2] decreases
C. right and the [SO2Cl2] increases
D. right and the [SO2Cl2] decreases
33. Consider the following equilibrium: NH3(g) + HCl(g) ⇄ NH4Cl(s) + energy
Which of the following will result in a decrease in the mass of NH4Cl?
A. adding NH3
B. removing
HCl
C. decreasing the volume
D. decreasing the temperature
34. Consider the following equilibrium: PCl3(g) + Cl2(g) ⇄ PCl5(g)
When 0.40 moles of PCl3 and 0.40 moles of Cl2 are placed in a 1.00 L container and allowed
to reach equilibrium, 0.244 mole of PCl5 are present. From this information, the value of the Keq is
A. 0.10
B. 0.30
C. 3.3
D. 10
Subjective
1. Concentrations of H2, I2, and HI in a mixture at equilibrium at 425 oC were found to be
1.52 x 10-2 M, 3.55 x 10-2 M, and 2.57 x 10-1 M respectively. Calculate the equilibrium constant.
H2(g) + I2(g)
⇄ 2HI(g)
Keq = (2.57 x 10-1)2 =
122
( 1.52 x 10-2)(3.55 x 10-2)
2. 4.00 moles of PCl5 are placed in a 2.00 L container and goes to equilibrium at 200 oC. If 0.60 moles of PCl5
are present at equilibrium, calculate the equilibrium constant.
PCl5(g) ⇄ PCl3(g) + Cl2(g)
I 2.00 0 0
C 1.70 1.70 1.70
E 0.30 1.70 1.70
Keq = (1.70)2 = 9.6
(0.30)
3. An equilibrium system has a Keq = 50 at 0 oC and a Keq = 80 at 20 oC.
a) As the temperature was increased, which direction did the reaction shift? Right
b) Is the reaction endothermic or exothermic? Endothermic
4. If the initial [H2] = 0.200 M and [I2] = 0.200 M and the Keq = 55.6 at 20 oC,
calculate the equilibrium concentration of all molecules.
H2(g) + I2(g) ⇄ 2HI(g)
I 0.200 0.200 0
C x x 2x
E 0.2 x 0.2
- x 2x
(2x)2 = 55.6
(0.2 x)2
2x = 7.457
0.2 x
x = 0.158 M
[HI] = 0.316 M [H2] = [I2] = 0.042 M
5. Consider the following data obtained for the following equilibrium:
Fe3+(aq) + SCN-(aq) ⇄ FeSCN2+(aq)
[Fe3+] [SCN-] [FeSCN2+]
Experiment 1 3.91 x 10-2 M 8.02 x 10-5 M 9.22 x 10-4 M
Experiment 2 6.27 x 10-3 M 3.65 x 10-4 M ?
Calculate [FeSCN2+] in experiment 2.
Keq = (9.22 x 10-4) =
2.940 x 102
(3.91 x 10-2)( 8.02 x 10-5)
Keq = (x) = 2.940
x 102
(6.27 x 10-3 M)(3.65 x 10-4
M)
x = 6.73 x 10-4 M
6. 1.60 moles CO, 1.60 moles H2O, 6.00 moles CO2, and 6.00 moles H2 are put in a
2.00 L container at 600 oC.
CO(g) + H2O(g) ⇄ CO2(g) + H2(g) Keq = 10.0
a) Show by calculation the reaction is not at equilibrium.
Trial Keq = (3.00)(3.00) = 14.06 >
10.0
(0.800)(0.800)
b) Which way will the reaction shift in order to achieve equilibrium?
Left
c) Calculate the equilibrium concentration of CO2.
CO(g) + H2O(g) ⇄ CO2(g) + H2(g)
I 0.800 0.800 3.00 3.00
C x x x x
E 0.800 + x 0.800
+ x 3.00 x 3.00 - x
[3.00 - x]2 = 10.0
[0.800 + x]2
[CO2] = x = 2.89 M