Redox Quiz #5 Application of Cells
1. The corrosion of iron can be prevented by attaching a piece of zinc to the iron
because the
A. iron acts as an anode
B. zinc reduces more readily than iron
C. electrons
flow from the zinc to the iron
D. iron ions form more readily than zinc ions
2. An iron spoon is electroplated with copper. The equation representing the
reduction reaction is
A. Cu2+(aq)
+ 2e- ® Cu(s)
B. Cu(s) ® Cu2+(aq) + 2e-
C. Fe2+(aq) + 2e- ® Fe(s)
D. Fe(s) ® Fe2+(aq) + 2e-
3. In an operating zinc-copper electrochemical cell, the oxidizing agent
A. loses electrodes at the anode
B. loses electrons to the cations
C. gains
electrodes at the cations
D. gains electrons from the anions
4. An example of electro refining is the
A. extraction of aluminum from bauxite
B. purification
of lead from an impure anode
C. recovery of zinc from a zinc sulphide solution
D. production of chlorine from a sodium chloride solution
5. Electroplating always involves the
A. oxidation of anions
B. reduction
of cations
C. reduction at the anode
D. oxidation at the cathode
6. Hydrogen and oxygen react to provide energy in a
A. dry cell
B. fuel
cell
C. alkaline cell
D. lead-acid storage cell
7. En electrolytic process is used to purify impure lead. The electrodes are
|
|
ANODE |
CATHODE |
|
A. |
carbon |
impure lead |
|
B. |
pure lead |
carbon |
|
C. |
pure lead |
impure lead |
|
D. |
impure
lead |
pure
lead |
1.0 M CuSO4
8. The half-reaction at the cathode is
A. Cu2+
+ 2e- → Cu(s)
B. 2SO42- → S2O82- + 2e-
C. H2O → ½ O2(g) + 2H+ + 2e-
D. 2H2O + 2e- → H2(g) + 2OH-
9. In the electrolysis of molten PbBr2 with inert electrodes, the products at the anode
and cathode are
Anode Cathode
A. Br2 H2
B. O2 Pb
C. Pb Br2
D. Br2 Pb
10. Under which conditions could an electrochemical cell provide 0.93V?
Anode Cathode
A. Cu Mg
B. Mg Cu
C. Ag Pb
D. Pb Ag
11. The reduction reaction in the above electrochemical cell is
A. Pb2+ + 2e- → Pb
B. Pb → Pb2+ + 2e-
C. Ag+ + e- →
Ag
D. Ag → Ag+ + e-
12. An industrial process involving electrolysis is the reduction of
A. water forming oxygen gas
B. water
forming hydrogen gas
C. sea water forming chlorine gas
D. sea water forming bromine liquid
13. To plate a nickel coin with copper
A. the
nickel coin must be the cathode
B. the cathode must be made of copper
C. the electrons must flow to the anode
D. the solution must contain nickel ions
14. Which of the following ions can be reduced from an aqueous solution
A. Ba2+
B. Al3+
C. Sn2+
D. Na+
15. The principal function of a fuel cell is to
A. produce fuel
B. electrolyze fuel
C. produce hydrogen
D. produce
electricity
16. If a piece of nickel is to be gold-plated using an electolytic process, which half-
reaction occurs at the cathode?
A. Ni → Ni2+ + 2e-
B. Ni2+ + 2e- → Ni
C. Au → Au3+ + 3e-
D. Au3+
+ 3e- → Au
17. Consider the following redox reaction
AS2O3 + 2NO3- + 2H2O + 2H+ → 2H3AsO4 + N2O3
In this reaction, nitrogen
A. loses electrons and increases in oxidation number
B. gains electrons and increases in oxidation number
C. loses electrons and decreases in oxidation number
D. gains
electrons and decreases in oxidation number
18. In an electrochemical cell, the cathode
A. is reduced
B. loses mass
C. is the reducing agent
D. is
the site of reduction
19. When 1.0 M NaI is electrolyzed, bubbles of gas form on one electrode and a
reddish-brown substance forms on the other. The half-reaction at the cathode is
A. 2I- → I2 +
2e-
B. Na+ + e- → Na
C. H2O + ½ O2 + 2H+ + 2e-
D. 2H2O
+2e- → H2 + 2OH-
20. Which of the following would prevent the corrosion of an iron nail?
A. Store the nail in acid.
B. Store the nail in dry air.
C. Store the nail in water.
D. Store the nail wrapped in nickel wire in a beaker of distilled water.
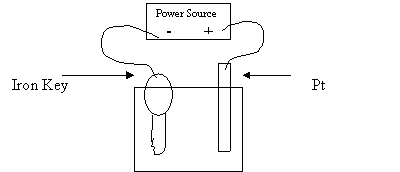
21. A student brought an old key to the Chemistry Lab to plate it with copper. He set
up a cell like the one in the following diagram:
1 M CuSO4
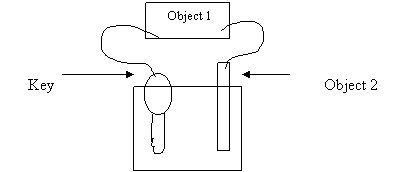
Which of the following combinations should produce the best result?
Object 1 Electron Flow Object 2
A. AC power supply towards the key Ag
D. voltmeter from the key Pt
22. Which of the following occurs when a solution of NiF2 is electrolyzed using
inert carbon electrodes?
A. The cathode dissolves.
B. Hydrogen gas is produced.
C. The pH of the solution decreases.
D. The Ni2+ concentration increases.
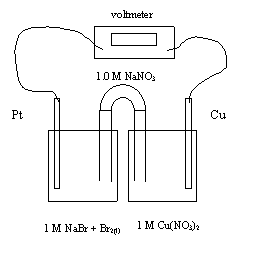
23. What is the cathode reaction for this cell?
A. Na+ + e- ® Na
B. Br2 + 2e- + ® 2Br-
C. 2Br- ® Br2 + 2e-
D. H2O ® 1/2O2 + 2H+ + 2e-
24. Which of the following best describes the movement of sodium ions and
electrons as the cell operates?
Na+ Ion Movement Electron Movement
A. towards the Cu towards the Pt
B. towards the Cu towards the Cu
C. towards the Pt towards the Cu
D. towards the Pt towards the Pt
25. What is the standard cell voltage?
A. -075 V
B. +0.62 V
C. +0.75 V
D. +1.43 V
26. Which of the following is an example of corrosion?
A. iron spontaneously oxidizing
B. sulphur spontaneously oxidizing
C. iron reducing
D. sulphur non-spontaneously oxidizing
27. Consider the following equation:
Co + SO42- + 4H+ ⇌ Co2+ + H2SO3 + H2O
Which statement is correct?
A. The sulphur is oxidized and the cobalt is reduced.
B. The cobalt is oxidized and the sulphur is reduced.
C. The hydrogen is reduced and the cobalt is oxidized.
D. The hydrogen is reduced and the oxygen is oxidized.
28. Which of the following occurs during the electrolysis of molten KCl?
A. Oxygen forms at the anode.
B. Potassium forms at the anode.
C. Chlorine forms at the cathode.
D. Potassium forms at the cathode.
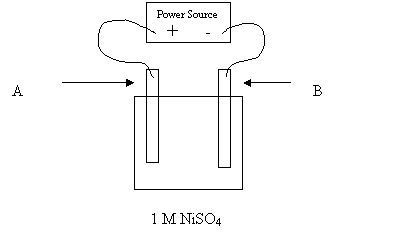
29. The above cell is constructed in order to nickel plate an object. For best results,
which of the following should be used for electrodes A and B?
Electrode A Electrode B
A. object pure nickel
B. pure nickel object
C. object any conductor
D. any conductor object
30. A student tries to use the above apparatus to nickel plate a zinc object. What will
happen if the student places the zinc object at A and the nickel electrode at B?
Electrode A Electrode B
A. Ni forms Ni dissolves
B. Zn dissolves Zn forms
C. Zn dissolves Ni forms
D. Bubbles form Bubbles form