Kinetics Workbook
for
Chemistry 12
Period Worksheets Quiz
- 1 Monitoring Reaction Rates WS 1 Q1
- 2 Factors that Change the Rate WS 2 Q2
- 3 Collision Theory WS 3 Q3
- 4 PE Diagrams WS 4 Q 4
- 5 Mechanisms WS 5 Q5
- 6 Lab: The Iodine Clock Reaction Internet Review
- 7 Review Practice Test 1
- 8 Review Practice Test 2
- 9. Test
This workbook will allow you to demonstrate your understanding of all aspects of the kinetics unit. The minimum expectation is that you do all of these questions by the due dates given by your teacher. Do the questions. Use your notes from class to assist you. Then after you have finished go to the web site to evaluate your work. Make a list of those things that you do not quite understand and bring them to class. Your instructor will review them. There are other things that you should do to prepare for the test at the end of the unit. Remember, what you put into this course is what you will get out. There is no substitute for consistent effort and hard work. If you cannot do a question, get some help before the end of the unit, you need to know, understand, and remember everything. Good luck! I know you can do well in this unit.
WS #1 Monitoring and Calculating Reaction Rates
1. Read the chapter from your textbook on Kinetics over the next week. “A” students should read it twice.
2. a) When measuring a property associated with a reactant in a reaction, does it increase or decrease?
2. b) When measuring a property associated with a product in a reaction, does it increase or decrease?
3. Give three ways to measure the rate of the following reaction. State the specific properties that you would monitor and include units (amount is not a specific property). State if each property would increase or decrease. Describe in each case how you would calculate the reaction rate.
2HNO3(aq) + Cu(s) → NO2(g) +
H2O(l) + CuNO3(aq)
The first one is done for you.
i) Mass of Cu Grams Decrease Rate = mass/time
ii)
iii)
4. Calculate the rate in units of (g Cu/min).
Mass of copper (g) 3.26 2.93 2.61
Time (min) 5.0 7.0 9.0
5. Calculate the
rate in units of (mole Cu/min).
6. Calculate the rate in moles HNO3
consumed per second (remember that 2 moles are consumed per 1 mole of Cu).
7. Calculate the rate in units of (g/sec)
for HNO3.
8. Calculate the rate in units of (mL NO2/sec).
2HNO3(aq) + Cu(s) → NO2(g) + H2O(l) +
CuNO3(aq)
Volume of NO2 (mL) 10.0 11.5 12.7
Time (sec) 0.00 5.00 10.00
9. Calculate
the rate in units of (L NO2/min).
10. Calculate the
rate in units of (moles NO2/min) at STP.
11. Calculate the rate in units of (moles HNO3/min) at STP (remember that 2 moles are consumed per 1 mole of NO2)
12. Calculate the rate of the following reaction:
2NO (g)
+ 2H2 (g)
→ N2 (g) +
2H2O (g)
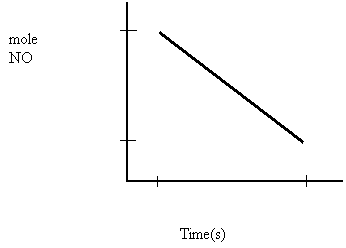
0.080
0.060
0.040
0.020
0.00 2.0
4.0 6.0 8.0
10.0 12.0
a) What is the rate in moles NO per second?
b) What is the rate in moles N2 per second?
c) What is the rate in grams NO per min?
d) What is the rate in grams N2 per hour?
13. Choose three properties that you could measure in order to monitor the rate of the following reaction.
Cu (s) + 2AgNO3 (aq) → 2 Ag (s) + Cu(NO3)2 (aq)
Property Unit of Measurement Change
i.
ii.
iii.
14. Calculate the rate of the following reaction in units of M/s:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Molarity of HCL (M) 0.612 0.813 1.05
time (seconds) 21.0 25.0 29.0
15. Calculate the rate of the following reaction in L/min:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Volume of H2 (L) 0.255 0.550 0.790
Time (min) 1.0 2.0 3.0
16. If 0.369 g of HCl is neutralized with 0.250 M NaOH in 25.0 seconds, what is the reaction rate in moles HCl /min.
WS # 2 Factors That Change The Reaction Rate
Homogeneous reactions
Reactants are in the same phase (aq), (g) , or (l) and are thoroughly mixed.
Heterogeneous reactions
Reactants are in the two or more phases and are not thoroughly mixed (two solids do not mix).
Classify as Homogeneous or Heterogeneous:
1. Zn(s) + 2 HCl(aq) → H2 (g) + ZnCl2 (aq)
2. Ag+(aq) + Cl-(aq)
→ AgCl (s)
3. H2(g) + F2(g)
→ 2HF(g)
4. 2Al(s) + 3I2(s)
→ 2AlI3(s)
The following four factors will increase the rate of a chemical reaction that is homogeneous:
1.
2.
3.
4.
The above four factors as well as the two below will increase the rate of a heterogeneous reaction:
5.
6.
For each reaction specifically describe all of the ways to increase the reaction rate
(i.e.. increase [H2]).
1. H2 (g) + F2 (g) → 2 HF (g)
2. HCl(aq) + NaOH(aq) → NaCl(aq) + H2O (l)
3. Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq)
4. State three examples of chemical reactions that are desired to be slow.
5. Give three examples of chemical reactions that are desired to be fast.
6. List all of the ways to increase the rate of the following reaction:
2H2O2(aq) → 2H2O(l) + O2(g)
I. Homogeneous reactions are generally faster than heterogeneous- the reactants are mixed better and therefore there are more collisions between reactant particles.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
is faster than
Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq)
II. Simple ionic reactions (where there are no bonds to break) are generally faster than more complex ionic reactions (where there are bonds to break).
Pb+2(aq) + 2Cl-(aq) → PbCl2(l)
is faster than
2Na+(aq) + 2ClO-(aq) → 2Na+(aq) + 2Cl-(aq) + O2(g)
Solid reactants are slower than gases, which are slower than aqueous.
1. Indicate the faster and slower reaction and explain why.
a) 2Al(s) + 3I2(s) → 2AlI3(s)
b) Ag+(aq) +
Cl-(aq) →
AgCl(s)
2. Indicate the faster and slower reaction and explain why.
a) 2Al(s) + 3I2(s) → 2AlI3 (s)
b) 2Na+(aq) + 2ClO-(aq) → 2Na+(aq) + 2Cl-(aq) + O2(g)
3. Indicate the faster and slower reaction and explain why.
a) 3Ba+2(aq) + 2PO4-3(aq) → Ba3(PO4)2(aq)
b) Cu(s) +
2Ag+(aq) → Cu+2 (aq) +
2Ag(s)
Ws # 3 Collision
Theory
1. Chemical reactions are the result of _________________ between reactant particles, where _________________ are broken and new ones form.
2. A successful collision requires _____________________ and __________________ .
3. Describe as fast, medium or slow. Explain!
i) 2 H2 (g) + O2 (g) → 2 H20 (l) (room temperature)
_______ _______________________________________________________
ii) 2 Ag+ (aq) + CO32- (aq) → Ag2CO3 (s)
_______ _______________________________________________________
iii) 2 HCl (aq) + Na2CO3 (aq) → CO2 (g) + 2 NaCl (aq) + H20 (l)
_______ _______________________________________________________
4. i) Describe how you would measure the rate of the reaction :
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g)
________________________________________________________________
ii) List four ways to increase the rate.
________________________________________________________________
________________________________________________________________
5. A 10 °C temperature increase frequently doubles the rate of a slow reaction because:
a) The temperature has doubled.
b) The PE of the colliding particle has doubled.
c) The KE of the colliding particle has doubled.
d) The fraction of particles with sufficient KE to react has doubled.
6. Both collisions A and B have the same KE. Which collision is successful and explain why.
Before Collision After Collision
![]()
![]()
![]()
A)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() B)
B)
________________________________________________________________
________________________________________________________________
7. Use the collision theory to explain how each factor increases the reaction rate.
i) Increasing temperature i) _________________
_________________
ii) Increasing [reactants] ii) _________________
iii) Increasing surface area (solid) iii) _________________
iv) Agitation of a heterogeneous reaction iv) _________________
v) Adding a catalyst v) _________________ _________________
8. Explain why collision A was successful while collision B was unsuccessful.
Before Collision After Collision
A)
![]()

B)
________________________________________________________________
________________________________________________________________
Explain each of the following using the collision theory. You need to explain each statement.
9.
A candle is not burning at room temperature
A match lights the candle
The candle
continues to burn
10.
H2O2 decomposes slowly at 20o C
KI is added and rapid decomposition begins
The temperature increases
11.
H2 and O2 in a balloon do not react
A spark ignites the balloon
An explosion results
12.
CH4 and O2 in a balloon do not react
A platinum gauze ignites the balloon
An explosion results
13. N2(g) + O2(g)
→ 2NO(g)
Even though there are more than four billion collisions per second between N and O the amount of product after a year is too small to detect. Using the collision theory, give two reasons why this reaction might be slow.
i)
ii)
14. Give two reasons why some collisions will not result in a chemical reaction.
i)
ii)
15. Give five reasons that might account for the following reaction having a high rate.
Ca (s) +
2HCl (aq) → CaCl2 (aq) + H2 (g)
i)
ii)
iii)
iv)
v)
16. C(s) + O2(g) → CO2(g)
List four ways the rate of the reaction could be increased.
i)
ii)
iii)
iv)
17. State the relationship between Activation energy and the rate of a reaction. Graph the relationship.

Rate
Activation Energy
18. State the relationship between Temperature and the rate of a reaction. Graph the relationship.

Rate
Temperature
19. State the relationship between Concentration and the rate of a reaction. Graph the relationship.

Rate
Molarity
20. Give three examples of reactions that are desired to be slow.
a)
b)
c)
21. Give three examples of reactions that are desired to be fast.
a)
b)
c)
22. List all of the ways to increase the rate of the reaction:
2 H2O2(aq) → 2 H2O(l) + O2(g)
23. Describe how you could measure the rate of the reaction above. State the property you would measure and describe how it changes. Draw a diagram to illustrate your answer.
24. Pick the fastest and the slowest reaction at 20 °C.
a) H2(g) + I2(g) → 2 HI(g)
b) 2 HCl(aq) + Na2CO3(aq) → CO2(g) + 2 NaCl(aq) + H2O(l)
c) Hg2+(aq) + 2 I -(aq) → HgI2(s)
25. H2 and O2 can exist at 20 °C for years without reacting. But when a small spark ignites the mixture it reacts explosively. Explain using the Collision Theory.
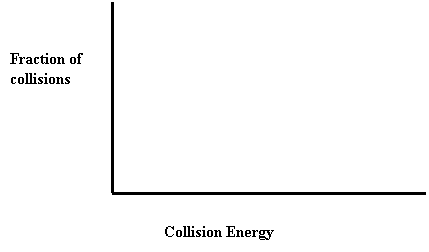
26. Draw a collision energy distribution diagram for a reaction where the y-axis is fraction of collisions and the x-axis is collision energy. Draw the Ea line showing about 10% of the collisions having sufficient energy. Draw the Ea line for the catalyzed reaction where 20% have sufficient energy.

27. Shade in the area of the collision energy distribution diagram showing those collisions that do not have the required energy to be successful at the temperature below.
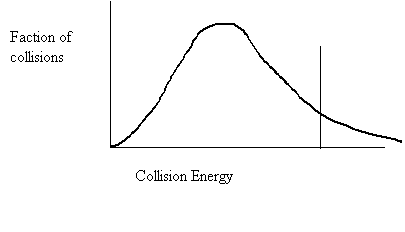
Ea
26. Shade in the area of the collision energy distribution diagram showing those collisions that do have the required energy to be successful at the temperature below. Redraw the curve at a higher temperature.
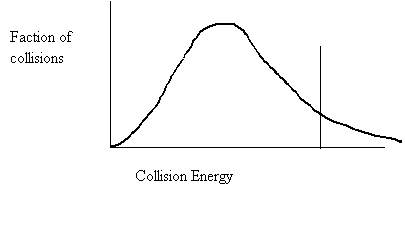
Ea
Kinetics - Descriptions
Use the collision theory to explain the
following. Each sentence must be explained with a statement from the collision
theory.
1. A unlit candle does not burn. It burns after being lit with a match. It continues to burn.
2. A solution is reacting very slowly to produce bubbles. KI is added and although it is not consumed in the reaction, it speeds up the reaction rate. The temperature increases. The rate increases even more.
3. Iron reacts slowly with HCl. Iron is replaced with Zn and a much more vigorous reaction rate occurs.
4. H2 and O2 can exist together for years at room temperature without reacting. A spark begins the reaction. An explosion results.
5. Dilute nitric acid shows little reaction with copper. Concentrated nitric acid vigorously reacts.
6. Water puts out a fire.
7. Paint prevents rusting.
8. A preservative in food slows rotting.
Ws # 4 Potential Energy Diagrams Worksheet
1. Draw the PE diagram showing the PE changes that occur during a successful collision of the exothermic reaction:
H2 + I2 → 2 HI + 250 kJ
The PE of the reactants = 400 kJ
Ea = 200 kJ
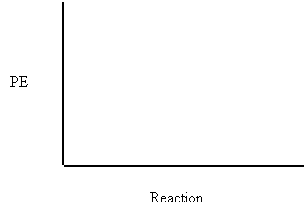
2. Draw the PE diagram showing the PE changes that occur during a successful collision of the endothermic reaction:
A + B + 200 kJ → C
The PE of the reactants = 200 kJ
The Activation Energy in the forward direction = 250 kJ
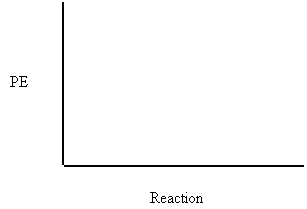
3. Write the following reaction in ΔH notation.
A + B + 200 kJ → C
4. Write the following reaction in Standard Notation.
H2 + I2 → 2 HI ΔH = -250 kJ
5. Write in Standard Notation.
2NI3 + 3BaCl2 → 2NCl3 + 3BaI2 ΔH = 175 kJ
6. Write in ΔH notation.
2AlBr3 + 3BaF2 → 2AlF3 + 3BaBr2 + 276 kJ
Draw the potential energy
diagram for the following reactions.
7. Potential energy of reactants = 250 kJ
Potential Energy of activated complex = 350 kJ
Potential Energy of the products = 300 kJ
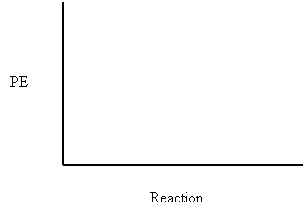
a) How does the potential energy change as the reaction proceeds?
b) How does the kinetic energy change as the reaction proceeds?
c) Is the reaction exothermic or endothermic?
d) What is the value of ΔH?
If a catalyst was added, what
would happen to the potential
energies of the:
e) Reactants?
f) Products?
g) Activated Complex?
h) If a catalyst were added what would happen to the rate?
Draw the potential energy
diagram for the following reactions.
8. Potential energy of reactants = 350 kJ
Activation Energy = 100 kJ
Potential Energy of the products = 250 kJ
a) How does the potential energy change as the reaction proceeds?
b) How does the kinetic energy change as the reaction proceeds?
c) Is the reaction exothermic or endothermic?
d) What is the value of ΔH?
If the concentration of the reactants was increased, what would happen to the energies of the:
e) Reactants?
f) Products?
g) Activated Complex?
h) What would
happen to the rate?
Draw the potential energy diagram for the following reactions.
9. Potential energy of reactants
= 200
kJ
Potential Energy of activated complex = 400 kJ
ΔH = 150 kJ
a) How does the potential energy change as the reaction proceeds?
b) How does the kinetic energy change as the reaction proceeds?
c) Is the reaction exothermic or endothermic?
d) What is the value of ΔH?
If the temperature was increased, what would happen to the energies of the:
e) Reactants?
f) Products?
g) Activated Complex?
h) What would happen to the rate?
10.
Potential energy of products = 50 kJ
Potential Energy of activated complex = 400 kJ
ΔH= -50 kJ
a) How does the potential energy change as the reaction proceeds?
b) How does the kinetic energy change as the reaction proceeds?
c) Is the reaction exothermic or endothermic?
d) What is the value of ΔH?
If the surface area of the reactants was increased, what would happen to the energies of the:
e) Reactants?
f) Products?
g) Activated Complex?
h) What would happen to the rate?
11. What is the only thing, other than changing the reaction that will change the potential energy diagram? Describe how it will affect the diagram and the rate.
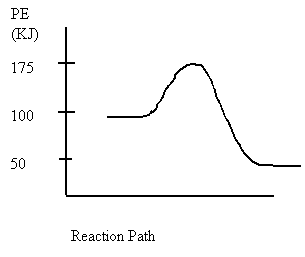
12. Label each interval on the potential energy diagram. a b c d e
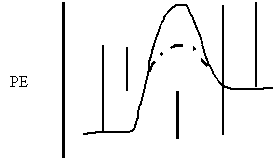
a)
b)
c)
![]() d)
d)
Reaction Path
e)
13. Label each interval on the potential energy diagram.
a b c d e
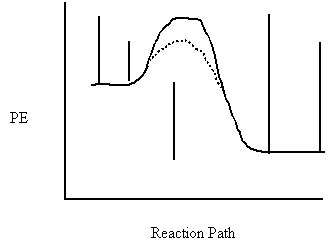
a)
b)
c)
d)
e)
Ws # 5 Mechanisms
1. OCl- + H2O → HOCl + OH-
HOCl + I- → HOI + Cl-
HOI + OH- → H2O + OI-
i) The net chemical equation is:
ii) The reaction intermediates are:
iii) The catalyst is:
2. Br2 → 2Br fast
Br + OCl2 → BrOCl + Cl slow
Br + Cl → BrCl fast
i) The net chemical equation is:
ii) The reaction intermediates are:
iii) The rate-determining step is
iv) If the concentration of Br2 is increased will the rate of the reaction increase? Explain your answer.
v) If the concentration of OCl2 is increased will the rate of the reaction increase? Explain your answer.
3. The mechanism for the catalytic decomposition of formic acid is shown below.
step 1 HCOOH + H+ → [HCOOHH]+
step 2 [HCOOHH]+ → [HCO]+ + HOH
step 3 [HCO]+ → CO + H+
The potential energy diagram is:
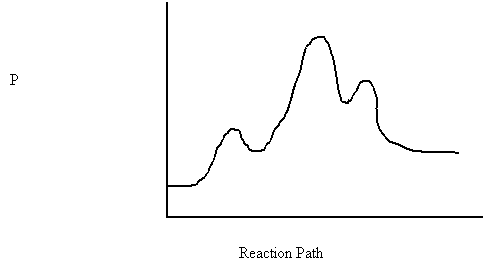
190
180
170
160
150
i) The catalyst is
ii) The rate determining step is
iii) ΔH =
iv) The reverse activation energy is
v) The enthalpy of [HCOOHH]+ is
vi) Is the reaction exothermic or endothermic?
vii) Which chemical formula has the greatest potential energy?
vii) Which chemical formula has the greatest kinetic energy?
ix) Does this reaction absorb or release kinetic energy?
4. Define and remember the following definitions.
Mechanism
Activation energy
Rate determining step
Catalyst
Reaction intermediate
Endothermic
Exothermic
Activated complex
ΔH
Reaction rate
5. The catalyzed decomposition of acetaldehyde has an overall reaction of:
CH3CHO → CH4 + CO . Determine step 2 of the reaction mechanism.
A proposed mechanism is:
step 1 CH3CHO + I2 → CH3I + HI + CO
step 2
6. The following reaction has an overall reaction of:
2Ce4+ + Tl+ → 2Ce3+ + Tl3+
Determine step 2 of the reaction mechanism.A proposed mechanism is:
step 1 Ce4+ + Mn+2 → Ce3+ + Mn3+
step 2
step 3 Mn4+ + Tl+ → Tl3+ + Mn2+
7. A reaction has a overall equation of: Br2 + OCl2 → BrOCl + BrCl . Determine step 3 of the mechanism. step 1 Br2 → 2Br
step 2 Br + OCl2 → BrOCl + Cl
step 3
List two intermediates:
8. Complete the following mechanism.
step 1 NO + Pt →
step 2 NOPt + NO → +
step 3 O2Pt → O2 + Pt
overall 2NO → N2 + O2
Identify the catalyst
Identify the two intermediates
9. Draw a collision energy distribution diagram for a reaction where the y-axis is fraction of collisions and the x axis is collision energy. Draw the Ea line showing about 10% of the collisions having sufficient energy. Draw the Ea line for the catalyzed reaction where 20% have sufficient energy.
Kinetics Quiz #1 Monitoring Reaction Rates
1. Consider
the following Reaction: HCl(aq)
+ NaOH(aq) →
H2O(1)
+ NaCl (aq) The rate of this reaction could be
determined by monitoring the change of concentration of :
A. H
+
B. Cl
-
C. Na
+
D. H2O
2. Consider the following reaction: 2Al (5) + 6HCl (aq) →
2AlCl 3(aq) + 3H 2(g)
A 0.040
mole piece of aluminum reacted completely in 20 s. The rate of formation of
hydrogen gas is :
A. 0.0013 mol/s
B. 0.0020 mol/s
C. 0.0030 mol/s
D. 0.0060 mol/s
3. Consider the following reaction: Zn(s) +
2HCl (aq)
→ ZnCl 2(aq) + H 2(g)
Solid
zinc was added to 1.0 M HCl. In 20.0 s.
the temperature of the container increased by 0.05oC and 25.00 ml of
H2 was produced. The rate was:
A. 0.5oC
B. 1.0 M HCl/s
C. 1.25 ml H2/s
D. 0.050 mol HCl/s
4. Consider the
following reaction: N 2(g)
+ 3H 2(g) →
2NH 3(g)
If the
rate of formulation of NH3 is 9.0 x 10–4 mol/s, then the
rate of consumption of N2 is:
A. 4.5 x 10-4 mol/s
B. 6.0 x 10-4 mol/s
C. 9.0 x 10-4 mol/s
D. 1.4 x 10-3 mol/s
5. In general,
the reaction rates double when the temperature is increased by 10oC
. The temperature of a reaction is
increased by 40oC. The rate of the reaction will be increased by:
A. 2
B. 4
C. 8
D. 16
6. Consider the following reaction: 2NO2(g) → 2NO(g) + O2(g)
Under
certain conditions, the rate of decomposition of NO2 is 3.2 x 10-3
mol/s. The rate of the formation of O2 is:
A. 1.6 x 10-3 mol/s
B. 3.2 x 10-3 mol/s
C. 4.8 x 10-3
mol/s
D. 6.4 x 10-3 mol/s
7. An 8.00 g
piece of magnesium was placed into 6.0 M HCl . After 25 s. 3.50 g of unreacted magnesium remained. The average
rate at which magnesium was consumed is:
A. 0.14 g/s
B. 0.18 g/s
C. 0.32 g/s
D. 4.50 g/s
8. The rate of a chemical reaction can be expressed in
A. grams per mole.
B. Energy consumed per mole.
C. volume of gas per unit time.
D. moles formed per liter of solution
9. Consider the following reaction at a constant temperature in
an open system:
MgCO3(s) +
2HCl(aq) → CO2(g) + H2O(l)
+ MgCl 2(aq)
Which of
the following properties could be used to determine reaction rate?
A. Mass of the system
B. Pressure of the gas
C. Concentration of H2O
D. Concentration of MgCO3
10. At 30oC
a 25.0 mL sample of bleach decomposes producing 50.0 mL of oxygen gas in 80
seconds. The rate of oxygen formation can be determined by the expression
A. 50.0 mL/80s
B. 50.0 mL/30oC
C. 25.0 mL/80s
D. 25.0 mL/30oC
11. A 25.0 mL
sample of hydrogen peroxide decomposes producing 50.0 mL of oxygen gas in 137
s. The rate of formation of O2 in
mL/min is
A. 0.182
mL/min
B. 0.365
mL/min
C. 10.9 mL/min
D. 21.9
mL/min
12. Consider the following
reaction: 2N2O5(g) → 4 NO2(g) +
02(g)
At a
certain temperature the rate of decomposition of N2O5 is 2.5 x 10-6 mol/s. The
rate of formation of NO2 is
A. 1.0 x 10-5 mol/s
B. 1.3 x 10-6 mol/s
C. 2.5 x 10-6 mol/s
D. 5.0 x 10-6 mol/s
Kinetics Quiz
#2 Factors
that Change The Reaction Rate
1. Which of the following reactions is the slowest at room
temperature?
A. Zn(s) + S(s) →
ZnS(s)
B. Ba2+(aq) + SO42-(aq)
→ BaSO4(s)
C. NH3(g) + HCl(g) → NH4Cl(g)
D. 2 Ag+(aq) + CO32-(aq)
→ Ag2CO3(s)
2. Dust
particles suspended in the air inside unheated grain elevators can sometimes react
explosively because the dust particles have a:
A. High kinetic energy
B. High activation energy
C. Catalytic effect on the reaction
D. Large surface area for the reaction
3. Consider the following reaction: 2H2O2(aq) →
2 H2O(l) + O2(g)
When 1.0
g of KI is added to the H2O2, bubbles of O2
are produced at an increased rate. When the reaction is complete, the mass of
KI is 1.0 g. The KI is a
A. Product
B. Catalyst
C. Reactant
D. Reaction Intermediate
4. Consider the following factors:
I.
Concentration of reactants.
II. Temperature
of reactants.
III. Surface area of reactants.
The
factors that affect the rate of a chemical reaction between two gases are
A. I and II only
B. I and III only
C. II and III only.
D. I, II, and III
5. Consider the following reactions:
I. N2(g) + O2(g) → 2NO(g)
II. 2Mg(s) + O2(g) → 2MgO(s)
III.
CaCO3(s) + 2H+(aq) →
Ca2+(aq) +
H2O(l) + CO2(g)
Increasing
the surface area will increase the reaction rate in
A. II only
B. I and III only
C. II and III only
D. I, II and III
6. An untreated sugar cube does not burn when
held over a lighted match. A sugar cube coated with cigarette ash readily
ignites and burns. All of the cigarette ash remains after the reaction. The
factor that caused this change in rate is the
A. Nature of reactants
B. Presence of a catalyst
C. Increase in surface area
D. Increase in concentration
7. Which combination of
factors will affect the rate of the following reaction?
Zn(s)
+ 2HCl (aq) →
ZnCl2(aq) + H2(g)
A. Temperature and surfaces only
B. Temperature and concentration only
C. Concentration and surface area only
D. Temperature, concentration, and surface
area
8. To increase the rate of a reaction there must be an increase
in
I frequency of successful collisions
II volume of reaction vessel
III pressure of the system
IV mass of the system
A. I only
B. I and III only
C. I, III and IV only
D. I, II, III and IV
9. Consider the
following reaction:
2MnO4-(aq) + 5C2O42-(aq) + 16H+(aq) → 2Mn2+(aq)
+ 10CO2(g) + 8H2O(l)
The rate
of decomposition of the oxalate ion is increased by
A. Adding NaOH
B. Removing CO2
C. Adding a catalyst
D. Decreasing the pressure.
10. Which of the
following factors affect the rates of both homogeneous and heterogeneous
reactions
I. Nature
of reactants
II.
Presence of a catalyst
III.
Temperature of system
IV
Concentration of reactants
A. I and IV only
B. II and III only.
C. II, III and IV only
D. I, II, III and IV
11. Which of the following
factors affects the rate of heterogeneous reactions only.
A. Nature of reactants
B. Temperature of system
C. Surface area of reactants
D. Concentration of reactants
12. Consider the following
reaction: 2S(s) + 302(g)
→ 2SO3(g) + heat
The rate
of this reaction could be increased by
A. Decreasing temperature
B. Adding a catalyst
C. Increasing the concentration of S(s)
D. Increasing the concentration of SO3(g)
Kinetics Quiz #3 Collision Theory
1. Which of the following are necessary for successful
collisions to occur?
I Favorable collision geometry
II
Sufficient Kinetic energy
III
Large ∆H
A. I only
B. I and II only
C. II and III only
D. I, II, and III
2. Collision theory states that
A. All collisions lead to chemical reactions
B. Most collisions lead to chemical
reactions
C. Very few reactions involve particle
collisions
D. Effective collisions lead to chemical
reactions
3. A catalyst increases the rate of a reaction by
A. Increasing the concentration of the
reactant(s)
B. Decreasing the concentration of the
reactant(s)
C. Increasing the activation energy of the
overall reaction
D. Decreasing the activation energy of the
overall reaction
4. Milk is
refrigerated in order to slow the rate of decomposition by bacterial action.
The decrease in reaction rate is due to
A. A decrease in surface area
B. A decrease in ∆H for the reaction
C. A decrease in the fraction of particles
possessing sufficient energy
D. The introduction of an alternate pathway
with greater activation energy
5. In general, a chemical reaction requiring a large activation
energy will proceed
A. At a fast rate
B. At a slow rate
C. Only at a low temperature
D. Only at low concentrations
6. Consider the following
reaction:
Mg(s) +
2HCl(aq)
→ MgCl2(aq) + H2(g)
As the temperature
of the above system is increased, the number of collisions
A. Increases but fewer are effective
B. Decreases and fewer are effective
C. Increases and more are effective
D. Decreases but more are effective
7. The minimum amount of energy needed to start a reaction is
called the
A. Activation energy
B. Energy of a reaction
C. Entropy of a reaction.
D. Reaction mechanism energy
8. When a lit match is touched to the wick of
a candle, the candle begins to burn. When the match is removed, the candle
continues to burn. In this reaction, the match
A. Behaves as a catalyst
B. Supplies activation energy
C. Is part of the rate-determining step
D. Lowers the activation energy barrier
9. Consider the
following collisions, each one occurring at the same temperature
Before
Collision Collision After Collision
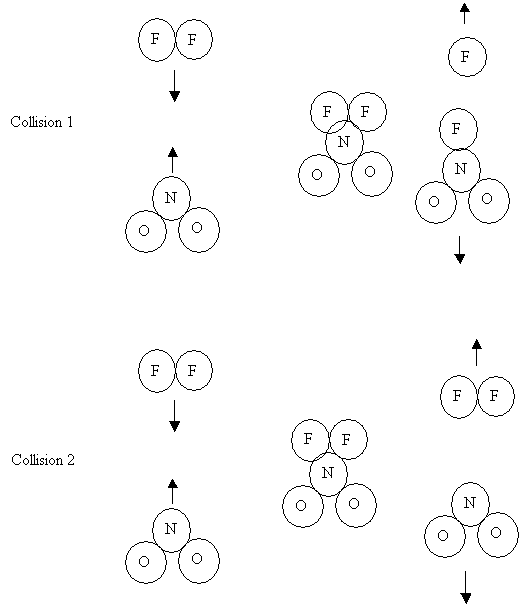
Before
Collision Collision After
Collision
Which one
of the following factors explains why collision one is successful while
collision two is not successful?
A. Catalyst
B. Geometry
C. Concentration
D. Kinetic energy
10. Consider the following factors
I Reactant
particles collide
II Sufficient
kinetic energy is present
III
A favorable geometry exists
IV
Catalysts are present
Which
combination of the above factors is required for successful collisions
A. I only
B. II and III only
C. I, II, and III only
D. I, II, III, and IV
11. To increase the rate of a reaction, there must be
A. Decrease in the frequency of collisions
B. An Increase in the frequency of
collisions.
C. A decrease in the frequency of successful
collisions
D. An increase in the frequency of
successful collisions
12. For collisions to be successful, reactants must have
A. Favorable geometry only
B. Sufficient heat of reaction only
C. Sufficient potential energy only
D. Sufficient kinetic energy and favorable
geometry
Kinetics Quiz 4 Potential Energy Diagrams
1. The addition of a catalyst to a reaction provides an alternative mechanism with
A. Lower activation energy and lower reaction rate
B. Lower activation energy and higher reaction rate
C. Higher activation energy and lower reaction rate
D. Higher activation energy and higher reaction rate
2. Consider the following Reaction: ½ N2(g) + ½ O2(g) → NO(g) ∆H = +90 kJ/mol NO
The correct equation including the heat term is
A. N2(g)
+ O2(g) + 90 kJ
→ 2NO(g)
B. N2(g)
+ O2(g) + 180 kJ
→ 2NO(g)
C. N2(g) + O2(g) → 2NO(g) +90kJ
D. N2(g) + O2(g) → 2NO(g) +180kJ
3. A forward reaction has activation energy of 50 kJ and a ∆H of –100 kJ.
The PE diagram, which describes this reaction, is
4. Consider the following potential energy diagram
The Activation energy for the forward reaction is
A. 25 kJ
B. 50 kJ
C. 75 kJ
D. 125 kJ
5. Consider the
following reaction: ½ H2(g) + ½ I2(g) → HI(g)
The activation energy for the formation of HI is 167 kJ and for the decomposition of HI is 139 kJ. The reaction for the formation of HI is
A. Exothermic and the ∆H = -28 kJ
B. Exothermic and the ∆H = +28 kJ
C. Endothermic and the ∆H = -28 kJ
D. Endothermic and the ∆H = +28 kJ
6. Consider the following potential energy diagram
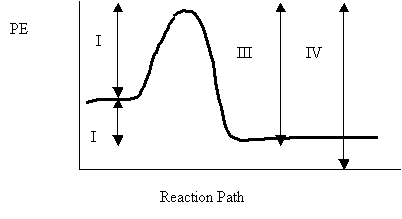
The energy interval the represents the activation energy for the reverse reaction is
A. I
B. II
C. III
D. IV
7. As reactant molecules approach each other
A. Heat is released
B. A reaction intermediate forms
C. Kinetic energy changes to potential energy
D. Potential energy changes to kinetic
8. Which of the following equations represents an endothermic reaction?
A. N204(g) +
59 kJ → 2NO2(g)
B. 2H2(g) + 02(g) → 2H2O(l) + 572 kJ
C. 2BrCl(g) – 29.3 kJ → Br2(g) + Cl2(g)
D. C(s) + O2(g) → CO2(g) ∆H = -394 kJ
9. Consider the following potential energy diagram
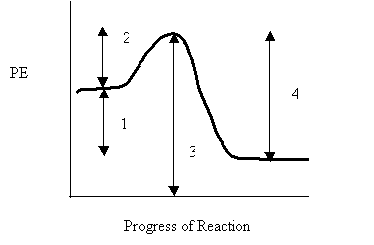
The interval representing ∆H for the reverse reaction is
A. 1
B. 2
C. 3
D. 4
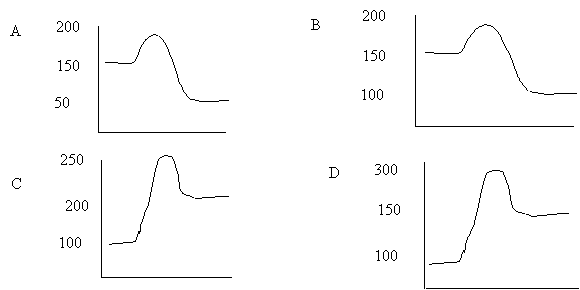
10. Which of the following corresponds to the fastest reaction at room temperature
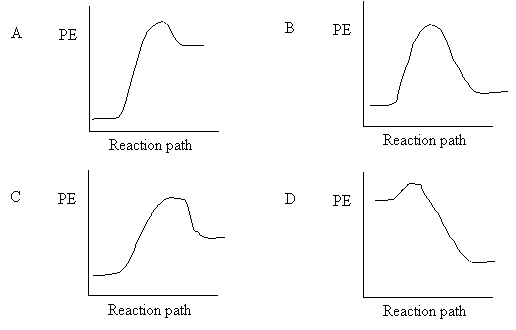
|
|
|
|
|
|
11. When a catalyst is added to a reaction, ∆H will
A. Increase slowly
B. Remain constant
C. Decrease slowly
D. Increase rapidly due to alternate pathway
12. Consider the following potential energy diagram that represents two different reactions. Which of the following statements is correct?

A B
A. Reactions A and B are both exothermic
B. Reactions A and B are both endothermic
C. Reaction A is exothermic and reaction B is endothermic
D. Reaction A in endothermic and reaction B is exothermic
13. Consider the following reaction: ½ H2(g) + ½ I2(g) → HI(g) ∆H = +28 kJ
The activation energy for the formation of HI is 167 kJ. the activation energy for the decomposition of HI is:
A. 28 kJ
B. 139 kJ
C. 167 kJ
D. 195
kJ
Kinetics Quiz
#5 Mechanisms
1. Consider the following reaction mechanism
Step 1: M + X → MX
Step 2: MX + A → D + X
The chemical species MX is a(n)
A. Catalyst
B. Inhibitor
C. Final Product
D. Reaction Intermediate
2. Consider the following reaction mechanism
Step 1: NO2 + NO2 → NO + NO3
Step 2: NO3 + CO → NO2 + CO2
The overall reaction is
A. 2NO2 → NO3 + NO
B. NO2 + CO → NO + CO2
C. NO3 + CO → NO2 + CO2
D. NO2 + NO3 + CO → NO3 + NO2 + NO + CO2
3. Consider the following reaction mechanism
Step 1: V3+ + Cu2+ → V4+ + Cu+ (slow)
Step 2: Cu+ + Fe3+ → Cu2+ + Fe2+ (fast)
The reaction intermediate is
A. Cu+
B. Cu2+
C. V3+
D. Fe3+
4. Consider the following reaction Mechanism
Step 1: H2O2 + I - → H2O + IO-
Step 2: H2O2 + IO - → H2O + O2 + I -
The reaction intermediate is
A. I -
B. IO -
C. H2O
D. H2O2
5. Consider the following potential energy diagram
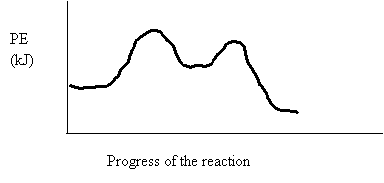
The above potential energy diagram represents an
A. Exothermic reaction involving one step
B. Exothermic reaction involving two steps
C. Endothermic reaction involving one step
D. Endothermic reaction involving two steps
6. Consider the following reaction mechanism
Step 1: NO2(g) + NO2(g) → NO(g) + NO3(g) (slow)
Step 2: NO3(g) + CO(g) → CO2(g) + NO2(g) (fast)
Which one of the following changes would result in the greatest increase in reaction rate
A. Increase [CO]
B. Decrease [NO]
C. Increase [NO2]
D. Increase [NO3]
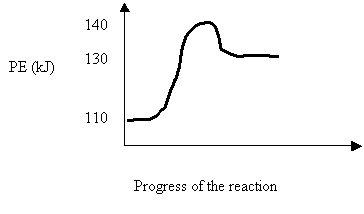
7. An uncatalyzed reaction was found to produce 40 kJ of energy in 10 mins. When catalyzed, the same reaction produced 40 kJ of energy in 2 mins. Which one of the following potential energy diagrams is consistent with the above data?
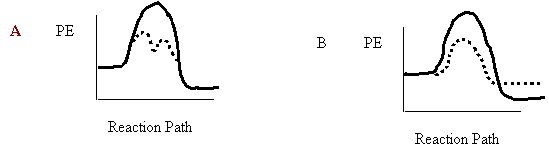
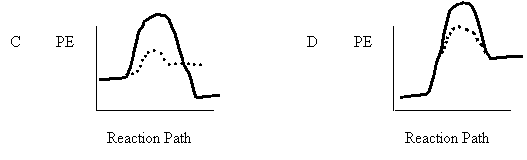
8. Consider the following reaction mechanism
Step 1: ICl + H2 → HI + HCl (slow)
Step 2: ICl + HI → HCl + I2 (fast)
The Species HCl is a
A. Product
B. Catalyst
C. Reactant
D. Reaction Intermediate
9. Consider the following reaction mechanism
Step 1: Cl(g) + O3(g) → ClO(g) + O2(g)
Step 2: O(g) + ClO(g) → Cl(g) + O2(g)
The Reaction intermediate is
A. Cl
B. O2
C. O3
D. ClO
10. In a reaction mechanism, the rate determining step is the
A. Fastest and has the lowest reaction rate.
B. Fastest and has the highest activation energy
C. Slowest and has the lowest activation energy
D. Slowest and has the highest activation energy
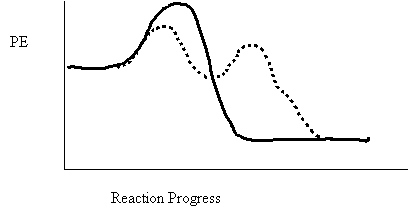
11. Select the true statement concerning the above potential energy diagram.
A. The catalyzed reaction has a larger ∆H
B. The uncatalyzed reaction has a larger ∆H
C. The catalyzed reaction has a greater rate of reaction
D. The uncatalyzed reaction has a greater rate of reaction
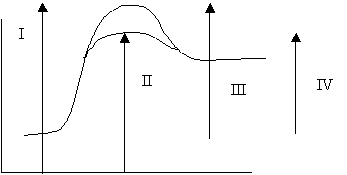
12. Which point on the diagram above represents the potential energy of the activated complex formed in the uncatalyzed reaction?
A. I
B. II
C. III
D. IV
13. Consider the following reaction
Step
1: NO(g) + O3(g)
→ NO2(g) + O2(g)
Step
2: O(g) + NO2(g) → NO(g) + O2(g)
The catalyst is
A. O2
B. O3
C. NO
D. NO2
14. Consider the following reaction mechanism
Step 1: N2O(g) → N2(g) + O(g)
Step
2: N2O(g) + O(g) → N2(g) + O2(g)
The reactant in the overall reaction is
A. O
B. O2
C. N2
D. N2O
15. Consider the following reaction
O3(g) + NO(g) ----- > NO2(g) + O2(g)
NO2(g) + O(g) ------ > NO(g) + O2(g)
The product in the overall reaction is
A. O2
B. O3
C. NO
D. NO2
Kinetics Web Review
1. Define the following:
Activation energy,
Mechanism
Activated Complex
Successful collision
Catalyst
Reaction rate
Enthalpy
Intermediate
Homogeneous reaction
Rate determining step
Heterogeneous reaction.
2. Na2CO3(aq) + 2HCl(aq) → CO2(g) + 2NaCl(aq) +H2O(l)
a) Give four ways to increase the rate of the reaction.
b) Give three properties that you could measure in order to determine the rate experiment.
3. Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq)
a) Give five specific ways to increase the rate.
4. In low light, H2 and Cl2 do not react at all. When exposed to UV light, they react explosively! Explain using the collision theory.
5. A mixture of KClO3 and C12H12O11 do not react at all at room temperature. A drop of H2SO4 starts the reaction, slowly at first, then it quickens into a flaming inferno. Explain using the collision theory.
6. Water puts out a fire. Explain using the collision theory.
7. A glowing splint re-ignites in pure O2. Explain using the collision theory.
8. Enzymes in the human body allow the oxidation of carbohydrates at 37 degrees Celsius.
9. Draw an exothermic PE diagram. Include a catalyst. Label the change in enthalpy, the forward and reverse activation energies and the activated complex.
10. Repeat the above for an endothermic reaction.
11. A student reacts CaCO3(aq) with excess HCl in an open container at a constant temperature. The equation for the reaction is:
CaCO3(s) + 2HCl(aq) → CO2(g) + CaCl2(aq) + H2O(l)
In terms of the collision theory, describe what will happen to the rate of the reaction as the reaction proceeds from the beginning to completion. Hint: what happens to the [HCl] as the reaction proceeds and what effect would that have on the rate.
12. A + WY → AWY Fast
AWY + HA → A2WY + H Slow
A2WY + HA → A3 + WY + H Fast
For the above reaction mechanism list the following:
a) The overall equation
b) A catalyst
c) Intermediates
d) Reactants
e) Products
Describe how each change affects the rate.
a) Increasing the concentration of A
b) Increasing the concentration of H
c) Increasing the concentration of HA
d) Removing WY completely
e) Decreasing the temperature
13. Describe the KE and PE changes as two molecules:
a) Approach to collide,
b) Form an activated complex, and
c) Form products in an exothermic reaction.
14. Draw the PE diagram for a mechanism with three steps. How many activated complexes are there? How many intermediates are there?
15. Describe as endothermic or exothermic.
a) 2H2 + O2 → 2H2O + 300kJ
b) NH4NO3(s) → NH4NO3(aq) ΔH = +150kJ
16. Change each equation in 15 from standard to ΔH notation or vice-versa.
17. Which reaction at room temperature is faster and why?
a) Pb2+ + 2Cl- → PbCl2 or
b) 2H2 + O2 → 2H2O
18. List three commercial catalysts (they are in your textbook)
19. Calculate the rate in moles/s.
|
Moles H2 |
10.0 |
15.0 |
21.0 |
24.0 |
|
Time (seconds) |
200 |
300 |
400 |
500 |
20. Indicate how each change will affect the rate of the reaction and the PE diagram and explain with the collision theory.
a) Increasing the temperature
b) Increasing the concentration of a reactant
c) Increasing the concentration of a product
b) Addition of a catalyst
21. X + Y → XY slow
XY + Z → XYZ fast
XYZ + W → XYW + Z fast
a) Identify intermediates, the catalyst and the rate-determining step.
b) If the concentration of X was increased will the rate increase?
22. You can only increase the surface area of a substance if it is in certain physical state. What is it?
23. Two solid reactants react. Is it a homogeneous or heterogeneous reaction?
24. What is the mathematical relationship between each of the following?
Reaction rate and activation energy
Reaction rate and reactant concentration
Reaction rate and temperature
25. Draw a collision energy distribution diagram for a reaction where the y axis is fraction of collisions and the x axis is collision energy. Draw the Ea line showing about 10% of the collisions having sufficient energy. Draw the Ea line for the catalyzed reaction where 20% have sufficient energy.

26. Shade in the area of the collision energy distribution diagram showing those collisions that do not have the required energy to be successful at the temperature below.
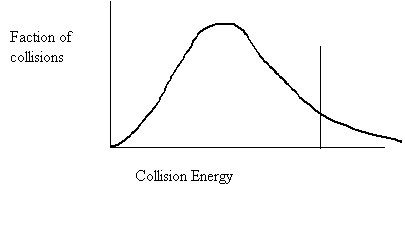
Ea
Practice Test # 1
1. Consider the following reaction mechanism:
step 1: M + X → MX
step 2: MX + A → D + X
The chemical species MX is a(n)
A. catalyst
B. inhibitor
C. final product
D. reaction intermediate
2. Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g)
At a certain temperature the rate of decomposition of N2O5(g) is 2.5 x 10-6 mol/s. The rate of formation of NO2 is
A. 1.0 x 10-5 mol/s
B. 1.3 x 10-6 mol/s
C. 2.5 x 10-6 mol/s
D. 5.0 x 10-6 mol/s
3. Which of the following factors affect the rates of both homogeneous and heterogeneous reactions.
I nature of the reactants
II presence of a catalyst
III temperature of system
IV concentration of reactants
A. I and IV only
B. II and III only
C. II, III, and IV only
D. I, II, III, and IV
4. Which of the following equations represents an endothermic reaction?
A. N2O4(g) + 59 kJ → 2NO2(g)
B. 2H2(g) + O2(g) → 2H2O(l) + 572 kJ
C. 2BrCl(g) -29.3 kJ →Br2(g) + Cl2(g)
D. 2H2(g) + O2(g) → 2H2O(l) ΔH = -572 kJ
5. Consider the potential energy diagram. The activation energy for the reverse reaction is
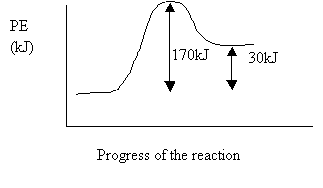
A. 30 kJ
B. 140 kJ
C. 170 kJ
D. 200 kJ
6. Consider the following mechanism: Step 1: Cl + O3 → ClO + O2
Step 2: O + ClO → Cl + O2
The reaction intermediate is
A. Cl
B. O2
C. O3
D. ClO
7. In a reaction mechanism, the rate determining step is the
A. fastest and has the lowest activation rate.
B. fastest and has the highest activation rate.
C. slowest and has the lowest activation rate.
D. slowest and has the highest activation rate.
8. A catalyst increases the rate of a reaction by
A. increasing the concentration of reactant(s).
B. decreasing the concentration of the reactant(s).
C. increasing the activation energy of the overall reaction.
D. decreasing the activation energy of the overall reaction.
9. Which of the following properties could be used to measure the rate of the following reaction in a open container. Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
A. mass of Zn
B. solubility of HCl
C. concentration of Cl-
D. colour of the solution
10. Consider the following potential energy diagram:
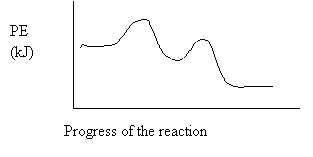
The above diagram represents an
A. exothermic reaction involving one step.
B. exothermic reaction involving two steps.
C. endothermic reaction involving one step.
D. endothermic reaction involving two steps.
11. Which of the following are necessary for successful collisions to occur?
I. Favourable geometry
II. Sufficient energy
III. Large ΔH
A. I only
B. I and II only
C. II and III only
D. I, II, and III
12. Consider the following reaction: 2H2O2(aq) → 2H2O(l) + O2(g)
When 1.0 g of KI is added to the H2O2, bubbles of O2 are produced at an increased rate, The KI is a
A. product
B. catalyst
C. reactant
D. intermediate
13. Consider the following
I. Frequency of successful collision
II. Volume of the reaction vessel
III. Pressure of the system
IV Mass of the system
To increase the rate of a chemical reaction there must be an increase in
A. I only
B. I and III only
C. I, III and IV only
D. I, II, III, and IV
14. Consider the following reaction mechanism:
Step1: ICl + H2 → HI + HCl slow
Step 2: ICl + HI → HCl + I2 fast
The species HCl is a
A. product
B. catalyst
C. reactant
D. reaction intermediate
15. Consider the following potential energy diagram:
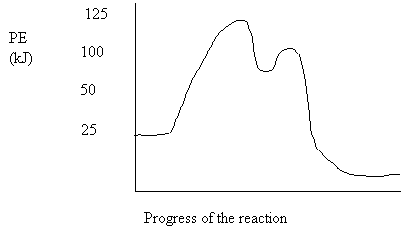
The activation energy in the forward direction is
A. 25 kJ
B. 50 kJ
C. 100 kJ
D. 125 kJ
16. Consider the following reactions:
I. N2 + O2(g) → 2NO(g)
II. Mg(s) + O2(g) → 2MgO(s)
III. CaCO3(s) + 2H+(aq) → Ca2+ (aq) + H2O(l) + CO2(g)
Increasing the surface area will increase the reaction rate in
A. II only
B. I and III only
C. II and III only
D. I, II, and III
17. Consider the following reaction mechanism:
Step 1: V3+ + Cu2+ → V4+ + Cu+
Step 2: Cu+ + Fe3+→ Cu2+ + Fe2+ slow
The reaction intermediate is
A. Cu+
B. Cu2+
C. V3+
D. Fe3+
18. The rate of a chemical reaction can be expressed in
A. grams per mole
B. energy consumed per mole
C. volume of gas per unit time
D. mole formed per litre of solution
19. Consider the following reaction:
2MnO4-(aq) + 5C2O42-(aq) 16H+(aq) → 2Mn2+(aq) + 10CO2(g) + 8H2O(l)
The rate of decomposition of the oxalate ion is increased by
A. adding NaOH.
B. removing CO2
C. adding a catalyst
D. decreasing the pressure
20. The minimum amount of energy needed to start a reaction is called the
A. activation energy.
B. energy of reaction.
C. entropy of reaction
D. reaction mechanism energy
21. An 8.00 g piece of magnesium was placed into 6.0 M HCl. After 25 s, 3.50 g of unreacted magnesium remained. The average rate at which magnesium was consumed is
A. 0.14 g/s
B. 0.18 g/s
C. 0.32 g/s
D. 4.50 g/s
22. In general rates double when the temperature is increased by 10 oC. The temperature of a reaction is increased by 40 oC. The rate will increase by a factor of
A. 2
B. 4
C. 8
D. 16
23. Consider the following factors
I. reactant particles collide
II. sufficient kinetic energy is present
III. a favourable geometry exists
IV. catalysts are present
Which combination of the above factors is required for all successful collisions?
A. I only
B. II and III only
C. I, II and III only
D. I, II, III, and IV
24. Consider the following reaction at constant temperature in an open system:
MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq)
Which of the following properties could be used to determine the reaction rate.
A. mass of the system
B. pressure of the gas
C. concentration of H2O
D. concentration of MgCO3
25. Which combination of factors will affect the rate of the following reaction?
MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq)
A. temperature and surface area only
B. temperature and concentration only
C. concentration and surface area only
D. temperature, concentration, and surface area only
26. As reactant molecules approach each other
A. heat is released
B. a reaction intermediate forms
C. kinetic energy changes into potential energy
D. potential energy changes into kinetic energy
Consider the following potential energy diagram for the next five questions.
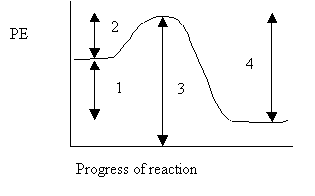
27. The interval representing ΔH for the reverse reaction is
A. 1
B. 2
C. 3
D. 4
28. The interval representing ΔH for the forward reaction is
A. 1
B. 2
C. 3
D. 4
29. The interval representing Ea for the reverse reaction is
A. 1
B. 2
C. 3
D. 4
30. The interval representing Ea for the forward reaction is
A. 1
B. 2
C. 3
D. 4
31. The interval representing the energy of the activated complex is
A. 1
B. 2
C. 3
D. 4
32. When a catalyst is added to a reaction, ΔH will
A. increase slowly
B. remain constant
C. decrease slowly
D. increase rapidly due to the alternate pathway
33. Consider the following reaction: Zn(s) + 2HCl(aq) → H2(g) + ZnCl2(aq)
Data for the reaction is shown below:
Time Mass of Zn (g) Volume of H2 (mL) Temperature (oC)
0 4.65 0 20
2 4.50 50 21
4 4.35 100 22
The rate of the reaction can be measured in units of
A. g/min
B. g/mL
C. min/mL
D. g/(mL)(oC)
34. When a lit match is touched to the wick of a candle, the candle begins to burn. When the match is removed, the candle continues to burn, the match,
A. behaves as a catalyst
B. supplies the activation energy
C. is part of the rate determining step
D. lowers the activation energy barrier
35. Consider the following reaction: 2NO(g) + O2(g) → 2NO2(g) + 112 kJ
ΔH for the above reaction is:
A. positive and the reaction is exothermic
B. negative and the reaction is exothermic
C. positive and the reaction is endothermic
D. negative and the reaction is endothermic
36. Consider the following reaction: 2S(s) + 3O2(g) → 2SO2(g) + heat
The rate of this reaction could be increased by
A. decreasing the temperature
B. adding a catalyst
C. increasing the concentration of S
D. decreasing the surface area of the S
37. Consider the following reaction: ½H2 + ½I2 → HI ΔH = +28 kJ
The activation energy for the formation of HI is 167 kJ. The activation energy for the decomposition of HI is
A. 28 kJ
B. 139 kJ
C. 167 kJ
D. 195 kJ
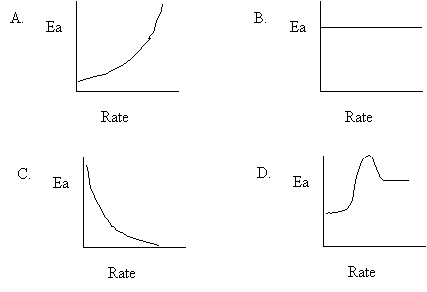
38. Some reactants are more reactive than others because of their activation energy Ea. What graph shows the relationship between Ea and rate.
39. The activated complex is a chemical species that is
A. stable and has low PE.
B. stable and has high PE.
C. unstable and has low PE.
D. unstable and has high PE.
40. As an activated complex changes into products,
A. potential energy changes into kinetic energy.
B. kinetic energy changes into potential energy.
C. kinetic energy changes into activation energy.
D. potential energy changes into activation energy.
Subjective
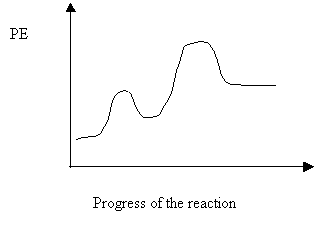
1. On the potential energy diagram above, clearly label the activation energy, heat of the reaction (∆H), and the energy of the activated complex.
2. Is the above reaction endothermic or exothermic in the forward direction?
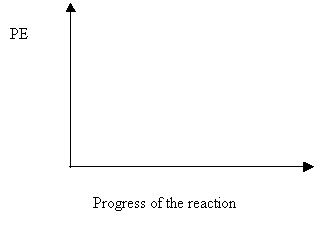
3. On the graph below, draw the potential energy diagram for an exothermic reaction and label the activation energy.
4. Nitric oxide (NO) is involved in the decomposition of ozone by the following mechanism:
Step 1: O3 + sunlight → O2 + O
Step 2: O3 + NO → NO2 + O2
Step 3: NO2 + O → NO + O2
a) Write the net equation for the decomposition reaction
b) Identify a catalyst
c) Identify a reaction intermediate
d) What is the function of sunlight in this reaction?
5. Consider the following reaction: 2NO + 2H2 → 2H2O + N2
a) Explain why the reaction is likely to involve more than one step.
b) A proposed
mechanism for the above reaction is:
Step 1: NO + H2 → N + H2O
Step 2: ?
Step 3: N2O + H2 → N2 + H2O
Write the equation for step 2.
6. Define the term activation energy.
7. The combustion of coal, C, produces carbon dioxide and water according to the following equation: C(s) + O2(g) → CO2(g) + 394 kJ
a) What is ∆H for this reaction?
b) Using the collision theory, explain why a lump of coal does not react with oxygen at room temperature and pressure.
c) Many coalmine disasters have resulted when a spark ignites coal dust in the air. Explain using the collision theory.
8. State two reasons why some collisions may not result in a chemical reaction.
9. A student wishes to monitor the rate of the following reaction:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Identify two different properties that could be used to monitor the rate of the reaction. Describe and explain the changes that would occur.
Property 1
Change and explanation
Property 2
Change and explanation
10. An experiment is done to determine the rate of the following reaction:
2Al(s) + 6HCl(aq) → 3H2(g) + 2AlCl3(aq)
1.00 g of Al is placed in a beaker
and allowed to react for 12.00 minutes
with 2.00 M HCl. If the rate of consumption
of HCl is 0.250 g/min, calculate the amount
of Al remaining.
Kinetics Practice Test # 2
1. Which of the following units could be used to express the reaction rate?
A. mL/s
B. mL/g
C. g/mL
D. mL/mol
2. Consider the reaction: Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
The rate of production of ZnCl2, can be increased by
A. decreasing the [HCl].
B. increasing the temperature
C. increasing the volume of H2.
D. decreasing the surface area of Zn.
3. The statement, the minimum energy needed for a successful collision, defines
A. enthalpy.
B. activation energy.
C. the ΔH of the reaction.
D. the activated complex.
4. As an activated complex changes to products,
A. potential energy changes to kinetic energy.
B. kinetic energy changes to potential energy.
C kinetic energy changes to activation energy.
D. potential energy changes to activation energy.
5. Which of the following is most likely to have the greatest rate at room temperature.
A. 2H2(g) + O2(g) → 2H2O(l)
B. 2Ag+(aq) + CrO42-(aq) → Ag2CrO4(s)
C. Pb(s) + 2HCl(aq) → PbCl2(aq) + H2(g)
D. CH4(g) + 2O2(g) → CO2(g) + H2O(g)
6 Consider the following PE diagram for an uncatalyzed and catalyzed reaction
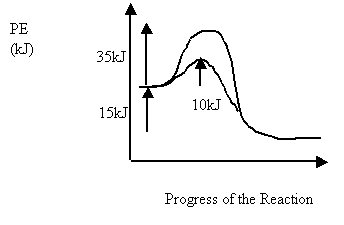
Which of the following describes the forward catalyzed reaction?
Activation Energy (kJ) ΔH (kJ)
A. 10 -15
B. 10 15
C. 25 -15
D. 25 15
7. A substance that increases the rate of a reaction without appearing in the equation for the overall reaction is a(an)
A. product
B. catalyst
C. reactant
D. intermediate
8. Activation energy can be described as the
A. energy of motion
B. energy of the activated complex.
C. energy difference between the reactants and the products.
D. energy difference between the reactants and the activated complex.
9. What effect does a catalyst have on a reaction?
A. It changes the ΔH of a reaction.
B. It increases the kinetic energy of the reactants.
C. It decreases the potential energy of the products.
D. It provides a reaction mechanism with a lower activation energy.
10. Consider the following reaction involving 1.0 g of powdered zinc:
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Trial Temperature (0C) Concentration of HCl
1 40 3.0
2 20 3.0
3 40 6.0
The rates in order of fastest to slowest are
A. 1, 2, 3
B. 2, 1, 3
C. 3, 1, 2
D. 3, 2, 1
11. Consider the following potential energy diagram for a reversible reaction:
![]()
![]()
![]()
Which of the following describes the system above?
Reaction Activation Energy (kJ) ΔH (kJ)
A. reverse 10 -20
B. reverse 10 -30
C. forward 30 +10
D. forward 20 +30
12. An activated complex is a chemical species that is
A. stable and has low PE.
B. stable and has high PE.
C. unstable and has low PE.
D. unstable and has high PE.
13. Consider the reaction: Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
At a certain temperature, 2.05 g Ca reacts completely in 30.0 seconds. The rate of consumption of Ca is
A. 0.00208 mol/min
B. 0.0833 mol/min
C. 0.102 mol/min
D. 5.00 mol/min
14. Increasing the temperature of a reaction increases the reaction rate by
I. increasing frequency of collision
II. increasing the kinetic energy of collision
III. decreasing the potential energy of the collision
A. I only.
B. I and II only.
C. II and III only.
D. I, II, and III.
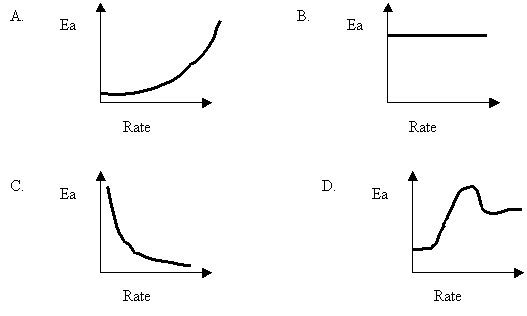
15. A certain reaction is able to proceed by various mechanisms. Each mechanism has a different Ea and results in a different overall rate. Which of the following best describes the relationship between the Ea values and the rates?
16. For collisions to be successful, reactants must have
A. favourable geometry.
B sufficient heat of reaction only.
C. sufficient potential energy only.
D. sufficient kinetic energy and favourable geometry.
17. Consider the following reaction: 1/2 H2(g) + 1/2 I2(g) → HI(g) ΔH = +28 kJ
The activation energy for the formation of HI is 167 kJ. The activation energy for the decomposition of HI is
A. 28 kJ
B. 139 kJ
C. 167 kJ
D. 195 kJ
18. Which of the following factors affects the rate of heterogeneous reactions only?
A. nature of the reactant
B. temperature
C. surface area of reactants
D. concentration of reactants
19. A 25.0 mL sample of hydrogen peroxide decomposes producing 50.0 mL of oxygen gas in 137 s. The rate of formation of O2 in mL/min is
A. 0.182 mL/min
B. 0.365 mL/min
C. 10.9 mL/min
D. 21.9 mL/min
20. Consider the following reaction mechanism:
step 1 2NO + H2 → N2 + H2O2
step 2 H2O2 + H2 → 2H2O
In this reaction H2 is a
A. product
B. catalyst
C. reactant
D. reaction intermediate
21. Which of the following properties could be used to measure the rate of the following reaction taking place in an open container?
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
A. mass of Zn
B. solubility of HCl
C. concentration of Cl-
D. colour of the solution
22. Consider the following reaction: N2 + 3H2 → 2NH3
The rate of formation of NH3 is 3.0 mole/min. The rate of consumption of H2 is:
A. 1.5 mole/min
B. 2.0 mole/min
C. 4.5 mole/min
D. 9.0 mole/min
23. Consider the following reaction mechanism:
Step 1 NO2 + NO2 → N2O4
Step 2 N2O4 + CO → CO2 + NO + NO2
In the overall reaction, N2O4 is a
A. product
B. catalyst
C. reactant
D. reaction intermediate
24. Consider the following mechanism:
Step 1 NO + O3 → NO2 + O2
Step 2 O + NO2 → NO + O2
The catalyst is
A. O2
B. O3
C. NO
D. NO2
25. Consider the following reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
The rate of this reaction increases when more Mg is added. This change is caused by the
A. addition of a catalyst
B. increase in surface area
C. change in the nature of the reactants
D. increase in concentration of reactants
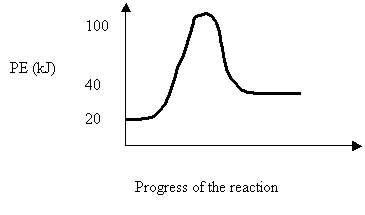
26. Consider the following PE diagram
The forward reaction can be described as
ΔH Ea Type
A. +20 80 endothermic
B. +20 60 exothermic
C. -20 80 exothermic
D. -20 100 endothermic
27. Consider the following reaction: HgO(s) → Hg(l) + 1/2O2(g)
The rate of this reaction can be expressed as
A. rate = [O2]1/2
B. rate = Δ[O2]/Δt
C. rate = Δ[Hg]/Δt
D. rate = Δ[HgO]/Δt
28. Which of the following would react most rapidly?
A. Powdered Zn in 1.0 M HCl at 25 0C
B. Powdered Zn in 2.0 M HCl at 40 0C
C. A lump of Zn in 2.0 M HCl at 25 0C
D. A lump of Zn in 1.0 M HCl at 40 0C
29. Addition of a catalyst to a reaction increases the rate because it
A. increases the value of ΔH
B. decreases the value of ΔH
C. provides an alternate mechanism with a lower Ea
D. provides an alternate mechanism with a higher Ea
30. When a collision occurs between two reactant species which possess between them the minimum kinetic energy a product does not always form. This may be a result of
A. low temperature
B. small surface area
C. low concentrations
D. unfavourable geometry
Subjective Section
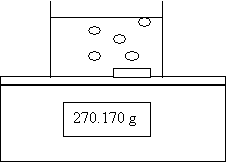
1. An experiment is done to determine the rate of the following reaction;
2Al(s) + 6HCl(aq) → 3H2(g) + 2AlCl3(aq)
H2
![]()
![]() The
following data are collected:
The
following data are collected:
Time (s) Mass of Beaker + Contents (g)
0.0 270.230
30.0 270.200
60.0 270.170
Calculate the rate of consumption of Al in moles/min
2. Define the term activation energy.
3. Define the word Activated complex.
4. Define the word mechanism.
5. Consider the following reaction mechanism
Step 1 ?
Step 2 H2 + Cl → HCl + H
Step 3 H + Cl2 → HCl + Cl
Step 4 Cl + Cl → Cl2
Overall H2 + Cl2 → 2HCl
a) Write the equation for step 1
b) Identify the reaction intermediate(s)
6. Consider the overall reaction: 4HBr + O2 → 2H2O + 2Br2
A proposed three-step mechanism is:
Step 1 HBr + O2 → HOOBr
Step 2 ?
Step 3 HBr + HOBr → H2O + Br2
Write the equation for step 2.
7. A student wishes to monitor the rate of the following reaction:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Identify two properties that could be used to monitor the rate of the reaction. Describe and explain the changes that would occur.
Property 1
Change and explanation
Property 2
Change and explanation
8. Carbon burns in air according to the following equation:
C(s) + O2(g) → CO2(g)
List four ways the rate of the above reaction could be increased.
9. Sketch the potential energy diagram for an endothermic reaction in the space below. On your diagram clearly label:
i) the energy of the activated complex
ii) the activation energy
iii) ΔH