Solubility Workbook
Period Worksheets Quiz
· 1. Solubility and Saturated Solutions. WS 1 1
· 2. Ion Concentration Calculations and Ionic Equations. WS 2-3 2
· 3. Solubility to Ksp. WS 4 3
· 4. Ksp to Solubilty and Size of Ksp. WS 5 4
· 5. Trial Ksp. WS 6 5
· 6. Separating Ions WS 7 6
· 7. Common Ion Effect and WS 8 7
· 8. Titrations and Max Ion Concentration WS 9 8
· 9. Review Web Review Practice Test 1
· 10. Review Practice Test 2
· 11. Test
The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the solubility unit. Ask yourself, “do I want to do well in this class?” If you are determined to be successful the minimum expectation that you should have for yourself is that you do all of these questions by the due dates given by your teacher. There are other things that you should do to prepare for the test at the end of the unit. Remember, what you put into this course is what you will get out. There is no substitute for consistent effort and hard work. If you can’t do a question, get some help before the end of the unit, you need to know, understand, and remember everything. Good luck! I know you can do well in this unit. Keep up the great work!
Worksheet #
1 Solubility
and Saturated Solutions
1. Define and give units for solubility.
2. Describe the relationship between the rate of dissolving and the rate of crystallization when a small amount of solute is added to an unsaturated solution.
3. Describe the relationship between the rate of dissolving and the rate of crystallization when a small amount of solute is added to a saturated solution.
4. Describe the relationship between the rate of dissolving and the rate of crystallization when a small amount of solute is added to a supersaturated solution.
5. Which of the above solutions would need to be prepared in order to determine the solubility of an ionic solution.
6. 2.65 g of Ba(OH)2 is dissolved in 70.0 mL of water to produce a saturated solution at 20 oC. Calculate the solubility in units of g/100 mL; g/L ; and M.
7. A beaker containing 100.0 mL of saturated BaCO3 solution weighs 159.60 g. The beaker is evaporated to dryness and weighs 56.36 g. The empty beaker weighs 24.33 g. Calculate the solubility in units of g/100 mL; g/ L; and M.
8. Write dissociation equations to represent the equilibrium present for a saturated solution of each ionic compound. Write the solubility product (Ksp expression) for each of the equilibrium systems. The first one is done.
a) Al2(SO4)3 ⇄
2Al3+ + SO42- Ksp
= [Al3+]2 [SO42-]3
b) FeCO3
c) Co2(SO4)3
d) Na3PO4
9. Write formula, complete ionic, and net ionic equations for each.
a) H2SO4(aq) + 2NaOH(aq)
→ Na2SO4 +
2HOH(l)
2H+ + SO42- +
2Na+ + 2OH- → 2Na+ +
SO42-
+ 2HOH(l)
H+ + OH- → HOH(l)
b) Mg(NO3)2(aq) + Na2CO3(aq) →
c) Al(NO3)3(aq) + (NH4)3PO4(aq) →
d) H3PO4(aq) + Ca(OH)2(aq) →
Worksheet # 2 Solubility
1. What is the concentration of each ion in a 10.5 M sodium silicate solution?
2. What is the concentration of each ion in the solution formed when 94.5 g of
nickel (III) sulphate is dissolved into 850.0 mL of water?
3. If 3.78 L of 0.960 M sodium fluoride solution is added to 6.36 L of 0.550 M calcium nitrate solution, what is the resulting concentration of [Ca+2] and [F-]?
4. What is the concentration of each ion in the solution formed when 94.78 g of
iron (III) sulphate is dissolved
into 550.0 mL of water?
5. If
the [F-] = 0.200 M,
calculate the number of grams AlF3 that would be dissolved in 2.00 L
of water.
6. If
the [SO42-] =
0.200 M in 2.0 L of Al2(SO4)3, determine the
[Al3+] and the molarity of the solution.
Dissociation
Equations Write dissociation equations for any chemicals, which dissociate when
dissolved in water:
1. HCl (aq) → H+ +
Cl- ionic compounds
dissociate
2. C6H12O6(S) → C6H12O6(aq) molecular compounds do not dissociate
3. Na2S (s)
4. Al(CH3COO)3 (s)
5. MgBr2 (s)
6. Na2CO3 (s)
7. C12H22O11 (s)
8. K3PO4
(s)
9. CH3OH
(l)
Net Ionic Equations
Write chemical
equations, total ionic equations and net ionic equations for each reaction. The
first one is done for you. (assume that all reactions occur):
1. Magnesium metal is placed in hydrochloric acid
Mg (s) + 2 HCl (aq) → MgCl2 (aq)
+ H2 (g)
Mg
(s) + 2 H+ (aq) + 2 Cl- (aq) → Mg2+
(aq) + 2Cl- (aq) +
H2 (g)
Mg
(s) + 2 H+ (aq) → Mg2+ (aq) + H2 (g)
2. Zinc metal is placed in silver nitrate solution
3. Barium chloride solution is added to lead (II) nitrate solution.
4. Sulphuric acid is added to strontium hydroxide solution.
5. Sodium carbonate solution is added to nickel (III) nitrate solution.
6. Aqueous chlorine is added to sodium bromide solution.
7. Nitric acid is added to strontium hydroxide solution.
Worksheet # 3 Solubility
1. Classify each as an ionic or molecular (covalent) solution.
NaCl (aq) __________ NH3 (aq) __________
CoCl2 (aq) _________ AgCl (aq) _________
CH3OH (aq) ________ HCl (aq) __________
NH4OH (aq) ________ I2 (aq) ___________
2. Define each:
a) unsaturated solution:
b) saturated solution:
c) solubility:
3. Describe how you would prepare a saturated solution.
4. Describe how you would determine the solubility of NaCl in water at 20oC.
5. In terms of equilibrium describe the difference between a saturated and unsaturated solution.
6. What is the effect of temperature on solubility?
7. 200.0 g of CoCl2 is dissolved in 500.0 mL of water at 0oC to form a saturated solution. What is the solubility of CoCl2 at 0oC in three different units?
8. In a saturated solution of CaCl2, a small amount of solid is present. Write a net ionic equation showing the equilibrium reaction. Write the solubility product (Ksp expression)
9. If you were given a saturated, unsaturated and supersaturated solution, how would you distinguish one from another?
a) Unsaturated solution:
b) Saturated solution:
c) Supersaturated solution:
10. Write the equilibrium equation and solubility product Ksp for each salt. The first one is done.
a) Ca(OH)2 (s) → Ca2+
+ 2OH- Ksp =
[Ca2+] [OH-]2
b) AgCl (s)
c) Na3PO4 (s)
d) (NH4)3PO4 (s)
e) Cu2SO4 (s)
f) Al(CH3COO)3 (s)
g) Ca3(PO4)2 (s)
Worksheet # 4 Solubility to Ksp
The Ksp is a measure of the solubility of an ionic salt. The larger the value of the Ksp, the greater is the solubility of the salt. You can only calculate a Ksp if the solution is saturated. Only saturated salt solutions are in equilibrium. You can calculate the Ksp from the solubility of a salt, since the solubility represents the concentration required to saturate a solution.
1. Calculate the Ksp for CaCl2 if 200.0g of CaCl2 is required to saturate 100.0 mL of solution.
2. Calculate the Ksp for AlCl3 if 100.0g is required to saturate 150.0 mL of a solution.
3. The solubility of SrF2 is 2.83 x 10-5 M. Calculate the Ksp.
4. The solubility of GaBr3 is 15.8 g per 100.0 mL. Calculate the Ksp.
5. The solubility of Ag2SO4 is 1.33 x 10-7g per 100.0 mL. Calculate the Ksp.
6. If 2.9 x 10-3 g of Ca(OH)2 is needed to saturate 250.0 mL of solution, what is the Ksp?
7. At a certain temperature, a 40.00 mL sample of a saturated solution of barium hydroxide, is neutralized by 29.10 mL of 0.300 M HCl. Calculate the Ksp of Ba(OH)2.
Calculate the concentrations of all ions in each solution.
8. 0.50 M Al2(SO4)3(aq)
9. 25.7 g (NH4)3PO4(aq) in 250.0mL H2O.
10. 210. g CoCl2 • 6H2O in 800.0 mL H2O.
Worksheet
# 5 Ksp
to Solubility
Calculate the solubility in M and g/L for each. Use the Ksp values found in your chart.
1. BaCO3
2. Fe(OH)2
3. PbCl2
4. How many grams of Mg(OH)2 are required to completely saturate 1.5 L of solution?
Review
1. If 200.0 g of MgCl2 is required to saturate 1.5 L of solution at 20 oC, calculate the Ksp.
2. If the Ksp for Al2O3 is 2.8 x 10-8, calculate [Al3+] and [O-2] in mol/L.
Worksheet # 6 Trial Ksp
1. Will a precipitate form if 200.0 mL 0.00020M Ca(NO3)2 is mixed 300.0 mL of 0.00030M Na2C03?
2. Will a precipitate form if 25.0 mL of
.0020M Pb(NO3)2
is mixed with 25.0 mL of 0.040M
NaBr.
3. Will a precipitate form if equal volumes
of 0.00020M Ca(NO3)2
is mixed with 0.00030M Na2C03?
4. Co(OH)2 Solubility = 3.0 x 10-3 g/L Calculate the Ksp at 250 C.
5. Ag2C2O4 Solubility = 8.3 x 10-4 M Ksp=?
6. SrF2 Calculate the solubility in (M).
7. Cu(IO3)2 Solubility Calculate the solubility in (g/L).
Worksheet # 7 Separation Positive Ions: Work
from top to bottom of solubility chart!!
1. Ag+ Mg2+ Ba2+
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out: Net Ionic equation:
2. Pb2+ Ba2+ Sr2+
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out: Net Ionic equation:
iii) Add:
Filter Out:
Net Ionic equation:
3. Cu+ Ca2+ Sr2+
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out: Net Ionic equation:
4. Be2+ Sr2+ Ag+
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add:
Filter Out:
Net Ionic equation:
5. Be2+ Ca2+ Pb2+
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out:
Net Ionic equation:
6. Calculate the Ksp for CaCl2,
if 50.0 g is required to saturate 25.0 mL of water.
7. Calculate the molar solubility of Mg(OH)2.
8. Will a precipitate form if equal volumes
of 0.00020 M Na2CO3 is mixed with
0.00020 M MgCl2.
9. Write the formula, complete, and net
ionic equation.
Formula Equation: CaCl2(aq) +
AgNO3(aq) →
Complete Ionic:
Net Ionic:
Separation of Negative Ions: Work from bottom to top of solubility
chart!!
1. SO32- OH- I-
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out: Net Ionic equation:
2. CO32- OH-
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
3. Br- S2- PO43-
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out: Net Ionic equation:
4. PO43- OH- S2-
i) Add:
Filter Out:
Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add:
Filter Out:
Net Ionic equation:
5. OH- S2- SO42-
i) Add:
Filter Out: Net Ionic equation:
ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out: Net Ionic equation:
6. S2- SO42- Cl-
i) Add:
Filter Out:
Net Ionic equation: ii) Add:
Filter Out:
Net Ionic equation:
iii) Add: Filter Out:
Net Ionic equation:
Common Ion Effect
Worksheet # 8
Consider the following equilibrium
system. PbCl2(s)
⇄ Pb2+(aq) +
2 Cl-(aq)
Describe what happens to the solubility of
PbCl2 after each of the changes.
Solubility
1. PbCl2(s) is added
2. Pb(NO3)2 is added
3. NaCl is added
4. H2O is added
5. AgNO3 is added
6. NaBr is added
Consider the following equilibrium
system. AgBr(s)
⇄ Ag+(aq) +
Br-(aq)
Describe what happens to the solubility of
AgBr after each of the changes are made.
Solubilty
7. AgBr(s) is added
8. Pb(NO3)2 is added
9. NaCl is added
10. H2O is added
11. AgNO3 is added
12. NaBr is added
13. Explain why more Zn(OH)2
dissolves when 3 M HCl is added to a saturated solution of Zn(OH)2. Start by writing the
correct equilibrium equation.
14. In an experiment, 0.1 M AgNO3
is added to 0.1 M NaCl, resulting in the formation of a white precipitate. When 0.1 M NaI is added to this
mixture, the white precipitate dissolves
and a yellow precipitate forms.
The formula for the white
precipitate is
The formula for the yellow
precipitate is
The net ionic equation for the first
equilibrium is
The net ionic equation for the formation
of the yellow precipitate is
Explain why the white precipitate
dissolves. Start by writing the equilibrium equation for the white precipitate, then, explain how
adding NaI affects this equilibrium.
Titrations and
Maximum Ion Concentration Worksheet # 9
1. In a titration 25.0 ml of a 0.250 M AgNO3 solution was used to precipitate out all of the Br- in a 200.0 ml sample. Calculate [Br-].
2. In a titration 26.5ml of 0.100M Pb(NO3)2 was used to precipitate out all of the Cl- in a 30.0 ml sample of water. Calculate [Cl-].
Maximum Ion Concentration
3. Calculate the maximum concentration of OH- that can exist in a 0.200M Mg(N03)2 solution.
4. Calculate the maximum concentration of CO3-2 that can exist in a 0.500M AgNO3 solution.
5. Calculate the maximum concentration of IO3- that can exist in a 0.200M Cu(N03)2 solution.
6. Calculate the maximum concentration of Ca2+ that can exist in a 0.200 M Na2C03 solution.
7. Calculate the minimum number of moles of Pb(NO3)2 required to start precipitation in 50.0 mL of 0.15 M ZnCl2.
8. In a titration 12.5 mL of 2.00 x 10-5 M HCl is required to neutralize 250.0 mL of saturated AgOH solution. Calculate the [OH-] and then determine the Ksp for AgOH.
9. When excess Ag2CO3(s) is shaken with 1.00 L of 0.200 M K2CO3 it is determined that 6.00 x 10-6 moles of Ag2CO3 dissolves. Calculate the solubility product of Ag2CO3.
Quiz #1 Solubility
and Saturated Solutions
1. To determine the solubility of solute in water, a solution must be prepared that is:
A. saturated
B. unsaturated
C. concentrated
D. supersaturated
2. From the list of salts below, how many are considered soluble at 25oC?
CuCl2 CaSO4 PbS Ag3PO4
A. zero
B. one
C. two
D. three
3. Which one of the following equilibrium systems is described by Ksp?
A. CaCO3(s) ⇄ CaO(s) + CO2(g)
B. CaCO3(s) ⇄ Ca2+(aq) + CO32- (aq)
C. Ca 2+(aq) + CO2-3(aq) ⇄ CaCO3(s)
D. Ca(OH)2(aq) + H2CO3(aq) ⇄ CaCO3(s) + 2H2O(l)
4. In a saturated solution, the rate of dissolving is
A. equal to zero
B. equal to the rate of crystallization
C. less than the rate of crystallization
D. greater than the rate of crystallization
5. A soluble magnesium salt is
A. MgSO3
B. MgCO3
C. Mg(NO3)2
D. Mg3(PO4)2
6. The equation that represents the equilibrium in a saturated solution of Fe2(SO4)3 is
A. Fe2(SO4)3(s) ⇄ 3Fe2+(aq) + 2SO43-(aq)
B. Fe2(SO4)3(s) ⇄ 2Fe2+(aq) + 3SO43-(aq)
C. Fe2(SO4)3(s)
⇄ 3Fe3+(aq)
+ 2SO42-(aq)
D.
Fe2(SO4)3(s)
⇄ 2Fe3+(aq)
+ 3SO42-(aq)
7. Which one of the following salts is soluble?
A. BaSO4
B. CaCO3
C. K3PO4
D. Fe(OH)2
8. The equation representing the equilibrium in a saturated solution of CaSO4 is
A. CaSO4(s) ⇄ Ca2+(aq)
+ SO42-(aq)
B. CaSO4(s) ⇄ Ca2+(aq)
+ S2-(aq) +
4O2-(aq)
C.
CaSO4(s) + H2O(l) ⇄ CaO(aq) + H2SO4(aq)
D.
CaSO4(s) + 2H2O(l) ⇄ Ca(OH)2(aq) +
H2SO4(aq)
9. Which of the following units is commonly used to describe solubility?
A. mL/s
B. g/oC
C. mol/L
D. oC/mol
10. Which of the following represents the equilibrium in a saturated solution of Cr2(SO4)3
A. Cr2(SO4)3(s) ⇄ Cr2+(aq)
+ SO43-(aq)
B. Cr2(SO4)3(s) ⇄ Cr3+(aq)
+ SO42-(aq)
C. Cr2(SO4)3(s) ⇄ 2Cr2+(aq)
+ 3SO43-(aq)
D. Cr2(SO4)3(s) ⇄ 2Cr3+(aq)
+ 3SO42-(aq)
Quiz #2 Ionic
Concentrations Calculations & Ionic Equations
1. A 200.0 mL solution contains 0.050 mol of Ba(NO3)2. The [NO3-] is
A. 0.050 M
B. 0.10 M
C. 0.25 M
D. 0.50 M
2. The Ksp expression for a saturated solution of Ca3(PO4)2 is
A. Ksp = [Ca2+][PO43-]
B. Ksp = [Ca2+]3[PO43-]2
C. Ksp = [3Ca2+][2PO43-]
D. Ksp = [3Ca2+]3[2PO43-]2
3. In 1.5 M (NH4)2SO4 , the ion concentrations are
A. [NH4+] = 1.5 M and [SO42-] = 1.5 M
B. [NH4+] = 1.5 M and [SO42-] = 3.0 M
C. [NH4+] = 3.0 M and [SO42-] = 1.5 M
D. [NH4+] = 3.0 M and [SO42-] = 3.0 M
4. The Ksp expression for Ca3(PO4)2 is
A. Ksp = [Ca2+]3[PO43-]2
[Ca3(PO4)2]
B. Ksp = [2Ca2+][3PO43-]
[Ca3(PO4)2]
C. Ksp = [Ca2+]3[PO43-]2
D. Ksp = [2Ca2+][3PO43-]
5. The solubility product expression for a saturated solution of Fe2(SO4)3 is
A. Ksp = [Fe3+]2[SO42-]3
B. Ksp = [2Fe3+][3SO42-]
C. Ksp
= [Fe3+]2[SO42-]3
[Fe2(SO4)3]
D. Ksp
= [2Fe3+][3SO42-]
[Fe2(SO4)3]
6. Molecular solutions do not conduct electricity because they contain
A. Molecules only
B. Cations and anions
C. Molecules and anions
D. Molecules and cations
7. If equal volumes of 0.2 M KBr and 0.2M FeSO4 are mixed, then
A. No precipitate will be observed
B. A precipitate of FeBr2 will be observed
C. A precipitate of K2SO4 will be observed
D. A precipitate of both K2SO4 and FeBr2 will be observed
8. Which of the following occurs when equal volumes of 0.20 M MgS and 0.20 M ZnSO4 are mixed?
A. A precipitate does not form
B. A precipitate of ZnS forms
C. A precipitate of MgSO4 forms
D. Precipitates of MgSO4 and ZnS form
9. In an experiment, 0.500 mol of Fe(NO3)3 is dissolved in water to produce a 2.00 L solution. The [NO3-] in this solution is
A. 0.250 M
B. 0.500 M
C. 0.750 M
D. 1.50 M
10. The complete ionic equation for the reaction between MgCl2(aq) and AgNO3(Aq) is
A. Ag+(aq) + Cl-(aq) → AgCl(s)
B. 2AgNO3(aq) + MgCl2(aq) → 2AgCl(s) + Mg(NO3)2(aq)
C. 2Ag+(aq) + Mg2+(aq) + 2NO3- (aq) + 2Cl-(aq) → MgCl2(s) + 2Ag+(aq) + 2NO3- (aq)
D. 2Ag+(aq) + 2NO3-
(aq) + Mg2+(aq) + 2Cl-(aq) → 2AgCl(s) + Mg2+(aq)+
2NO3- (aq)
11. Which one of the following would form an ionic solution when dissolved in water?
A. I2
B. CH3OH
C. Ca(NO3)2
D. Cl2H22O11
12. If the solubility of Pb (OH) 2 is 0.155g/L, then the concentration of each ion in a saturated solution of a Pb (OH) 2 is
A. [Pb2+] = 0.155 g/L and [OH-] = 0.155g/L
B. [Pb2+] = 0.052 g/L and [OH-] = 0.103g/L
C. [Pb2+] = 6.43 x 10-4 M and [OH-] = 1.29 x 10-3 M
D. [Pb2+] = 6.43 x 10-4 M and [OH-] = 6.43 x 10-4 M
13. The Ksp expression for calcium hydroxide is
A. Ksp = [Ca2+][OH-]2
B. Ksp = 1
[Ca2+][OH-]2
C. Ksp = [Ca2+][2OH-]2
D. Ksp = 1
[Ca2+][2OH-]2
14. A precipitation reaction occurs when equal volumes of 0.2 M Pb(NO3)2 and 0.2 M
KI are mixed. The net ionic equation for this reaction is
A. Pb2+(aq) + 2I-(aq) → PbI2(s)
B. PbI2(s) → Pb2+(aq) + 2I-(aq)
C. K+(aq) + NO3-(aq) → KNO3(s)
D. KNO3(s) → K+(aq) + NO3-(aq)
15. When dissolved in water, which of the following is an ionic solution?
A. O2
B. CH4
C. CaCl2
D. C12H22O11
16. In a 200.0 mL sample of 0.030 M Na3PO4, the [Na+] is
A. 0.006 M
B. 0.010 M
C. 0.018 M
D. 0.090 M
Quiz #3 Solubility
to Ksp
1. In a saturated solution of manganese (II) hydroxide, Mn(OH)2, and [Mn2+] equals
4.5 x 10-5 M. therefore, the Ksp of Mn(OH)2 is
A. 9.1 x 10-14
B. 3.6 x 10-13
C. 2.0 x 10-9
D. 4.1 x 10-9
2. The solubility of manganese (II) sulphide is 1.7 x 10-7 M at 25oC. The solubility product constant is
A. 2.9 x 10-14
B. 1.7 x 10-7
C. 3.4 x 10-7
D. 4.1 x 10-4
3. The compound Ag2S has a solubility of 1.3 x 10-4 moles per litre at 25oC. The Ksp for this compound is
A. 2.2 x 10-12
B. 8.8 x 10-12
C. 1.7 x 10-8
D. 3.4 x 10-8
4. The solubility of barium oxalate, BaC2O4, is 4.8 x 10-4 M. The value of Ksp is
A. 2.3 x 10-7
B. 4.8 x 10-4
C. 2.4 x 10-4
D. 2.2 x 10-2
5. The solubility of MnS is 4.8 x 10-7 M, at 25oC. The Ksp value is
A. 2.3 x 10-13
B. 4.8 x 10-7
C. 9.6 x 10-7
D. 6.9 x 10-4
6. At 25oC, the solubility of an unknown compound is 7.1 x 10-5 M. the compound is
A. CuI
B. AgI
C. CaCO3
D. CaSO4
7. The solubility of barium fluoride is 3.6 x 10-3 M. the solubility product constant is
A. 4.7 x 10-8
B. 1.9 x 10-7
C. 1.3 x 10-5
D. 2.6 x 10-5
8. At a certain temperature, the solubility of BaF2 is 7.4 x 10-3 moles per litre. The Ksp of BaF2 is
A. 1.6 x 10-6
B. 5.5 x 10-5
C. 1.1 x 10-4
D. 7.4 x 10-3
Quiz #4 Ksp to Solubility and Size of Ksp
1. Identify the most soluble sulphide
A. HgS, Ksp = 1.6 x 10-54
B. PbS, Ksp = 7.0 x 10-29
C. FeS, Ksp = 3.7 x 10-19
D. MnS, Ksp = 2.3 x 10-13
2. Saturated solutions of Na2S, CuS, SnS2, and Al2S3 are prepared at 25oC. The [S2-] will be greatest in the solution of
A. Na2S
B. CuS
C. SnS2
D. Al2S3
3. A solution of AgNO3 is slowly added to a mixture containing 0.10 M I-, Cl-, Br-, and IO3-. The precipitate, which forms first, is
A. AgI
B. AgCl
C. AgBr
D. AgIO3
4. The [OH-] is measured to be 3.3 x 10-3 mol/L in a 100.0 mL sample of saturated solution of Al(OH)3. The solubility of Al(OH)3 is
A. 1.1 x 10-4 mol/L
B. 3.3 x 10-4 mol/L
C. 1.1 x 10-3 mol/L
D. 3.3 x 10-3 mol/L
5. The [SO24-] in a saturated solution of PbSO4 is (Ksp = 1.1 x 10-8)
A. 1.2 x 10-16 M
B. 5.5 x 10-9 M
C. 1.1 x 10-8 M
D. 1.0 x 10-4 M
6. The least soluble in salt water is
A. BaS
B. AlCl3
C. CaSO3
D. ZnSO4
7. The solubility of AgBrO3 is
A. 2.8 x 10-9 M
B. 5.3 x 10-5 M
C. 1.1 x 10-4 M
D. 7.3 x 10-3 M
8. At 25oC, the solubility of Mg (OH)2 is
A. 1.1 x 10-32 M
B. 5.6 x 10-12 M
C. 2.4 x 10-6 M
D. 1.1 x 10-4 M
9. A student evaporated 200.0 mL of a saturated solution of SrCrO4 to dryness. The residue contained 1.2 x 10-3 mol SrCrO4. The solubility of SrCrO4 is
A. 1.4 x 10-6 M
B. 3.6 x 10-5 M
C. 2.4 x 10-4 M
D. 6.0 x 10-3 M
10. The solubility of magnesium carbonate is
A. 9.3 x 10-5 M
B. 3.4 x 10-6 M
C. 6.8 x 10-6 M
D. 2.6 x 10-3 M
11. A saturated container of NiCO3 was evaporated to dryness. A 250.0 mL sample was found to contain 1.1 x 10-2 g NiCO3. The molecular mass of NiCO3 is 118.7 g/mol. The molar solubility of NiCO3 is
A. 9.3 x 10-5 M
B. 3.7 x 10-4 M
C. 4.4 x 10-2 M
D. 1.4 x 10-7 M
12. Which of the following is the least soluble in water at 25oC ?
A. CaCO3
B. BaSO4
C. CuSO4
D. MgSO4
13. Which of the following salts has the lowest solubility?
A. Copper (I) chloride
B. Ammonium sulphide
C. Potassium hydroxide
D. Mercury (II) sulphate
Quiz #5 Trial Ksp
1. In an experiment, a student mixes equal volumes of 0.0020 M Pb2+ ions with 0.0040 M I- ions. The trial ion product is
A. 4.0 x 10-9
B. 3.2 x 10-8
C. 1.3 x 10-7
D. 8.0 x 10-6
2. In an experiment, 20.0 ml of 0.0060 M CaCl2 and 20.0 mL of 0.0050 M NaSO4 are mixed together. The trial ion product (trial Ksp) is
A. 7.5 x 10-6 and a precipitate will form
B. 7.5 x 10-6 and a precipitate will not form
C. 3.0 x 10-5 and a precipitate will form
D. 3.0 x 10-5 and a precipitate will not form
3. In a saturated solution of Zn(OH)2, the [Zn2+] is
A. Less than 0.10 M
B. More than 10.0 M
C. More than 0.10 M but less than 1.0 M
D. More than 1.0M but less than 10.0 M
4. When 0.20 M Al2(SO4)3 is added to an equal volume of 0.20 M CaCl2,
A. AlCl3 precipitates
B. CaSO4 precipitates
C. AlCl3 and CaSO4 precipitate
D. no precipitate forms
5. When a student mixes equal volumes of 0.20 M Na2S and 0.20 M Sr(OH)2.
A. No precipitate forms
B. A precipitate of only SrS forms
C. A precipitate of only NaOH forms
D. Precipitates of both NaOH and SrS form
6. When solutions of Pb(NO3)2 and NaCl are mixed, the trial ion product (Trial Ksp)
is 9.8 x 10-6. Which of the following statements is true?
A. A precipitate forms because Ksp > 9.8 x 10-6
B.
A precipitate forms because Ksp
< 9.8 x 10-6
C. A precipitate does not form because Ksp < 9.8 x 10-6
D. A precipitate does not form because Ksp > 9.8 x 10-6
7. When equal volumes of 0.060 M AgNO3 and 0.00090 M NaBrO3 are mixed, the trial ion product (TIP) is
A. Less than Ksp and a precipitate forms
B. Greater than Ksp and a precipitate forms
C. Less than Ksp and no precipitate forms
D. Greater than Ksp and no precipitate forms
8. The mixture that could produce a precipitate of two compounds is
A. 0.2 M HgSO4 and 0.2 M FeCl2
B. 0.2 M AgNO3 and 0.2 MgCl2
C. 0.2 M K2CO3 and 0.2 CuSO4
D. 0.2 M ZnSO4 and 0.2 Ba(OH)2
9. Which of the following has a solubility of less than 0.10 M?
A. SrS
B. SrCl2
C. SrSO4
D. Sr(OH)2
10. When equal volumes of 2.0 M Pb(NO3)2 and 2.0 M KCl are mixed
A. A precipitate forms because trial ion product < Ksp
B. A precipitate forms because trial ion product > Ksp
C. A precipitate does not form because trial ion product < Ksp
D. A precipitate does not form because trial ion product > Ksp
Quiz #6 Separating Ions
1. During a lab on qualitative analysis, an unknown solution containing one cation was analyzed and the following data were collected:
|
0.2 M Anions Added to the Unknown Solution |
Observation |
|
S2- |
no precipitate |
|
SO42- |
precipitate |
|
OH- |
precipitate |
|
CO32- |
precipitate |
Which one of the following cations is found in the unknown solution?
A. Mg2+
B. Be2+
C. Sr2+
D. Ba2+
2. Which of the following could be used to precipitate both Mg2+ and Ca2+ from hard water?
A. Lithium sulphate
B. Sodium phosphate
C. Potassium sulphide
D. Ammonium chloride
3. A reagent that may be used to separate Cl- from S2- by precipitation is
A. KNO3
B. AgNO3
C. Pb(NO3)2
D. Al(NO3)3
4. The precipitate formed when equal volumes of 0.2 M Sr(OH)2 and 0.2 M MgS are mixed is
A. SrS
B. Mg(OH)2
C. a mixture of Mg(OH)2 and SrS
D. a mixture of Sr(OH)2 and MgS
5. Which of the following ions could be used to separate Cl-(aq) from SO42-(aq) by precipitation?
A. Ag+
B. Ca2+
C. NH4+
D. Pb2+
6. Which of the following ions could be added to an aqueous mixture containing Pb2+ and Ba2+ to separate the ions by precipitating one of them?
A. I-
B. NO3-
C. PO43-
D. SO42-
7. Which of the following would precipitate the Ca2+ and Mg2+ found in hard water?
A. S2-
B. PO43-
C. SO42-
D. CH3COO-
8. A solution containing an unknown cation was added to three solutions and the f following observations were recorded:
|
SOLUTION |
OBSERVATION |
|
NaI |
no precipitate |
|
Na2SO4 |
precipitate |
|
NaOH |
no precipitate |
The unknown cation is
A. Pb2+
B. Sr2+
C. Ca2+
D. Ag+
9. To remove Mg2+ from a solution by precipitation, a student should add
A. NaI
B. KOH
C. Li2SO4
D. (NH4)2S
10. Which of the following could be used to separate Pb2+ from Ba2+ by precipitation?
A. Na2S
B. NaOH
C. Na2CO3
D. Na3PO4
11. A student wishes to identify an unknown cation in a solution. A precipitate does not form with the addition of SO42-, but does form with the addition of S2-. Which of the following is the unknown cation?
A. Ag+
B. Mg2+
C. Ca2+
D. Cu2+
12. A solution contains CO32- and OH-. Separation of these two anions by selective precipitation is accomplished by first adding Sr(NO3)2 solution, then filtering and finally adding to the filtrate a solution of
A. HNO3
B. RbNO3
C. NH4NO3
D. Zn(NO3)2
13. A nitrate solution containing an unknown cation is added to each of the following three test tubes.
1.0
M NaOH 1.0
M Na2S 1.0
M Na2SO4
![]()
![]()
![]()
A precipitate forms in one test tube only. The unknown cation is
A. Ag+
B. Ca2+
C. Sr2+
D. NH4+
14. A solution containing a single unknown cation is added to three test tubes. the following anions were added and observations were recorded.
|
TEST TUBE |
ANION ADDED |
OBSERVATION |
|
1 |
SO42- |
precipitate |
|
2 |
S2- |
precipitate |
|
3 |
OH- |
precipitate |
The solution contains
A. Sr2+
B. Ag+ or Pb2+
C. Ca2+ or Ba2+
D. K+, NH4+ or H+
Quiz #7 Common Ion Effect
1. Consider the following equilibrium: CaCO3(s) ⇄ Ca2+(aq) + CO32-(aq)
Which of the following reagents, when added to the equilibrium system, would cause more CaCO3 to dissolve?
A. KNO3(s)
B. CaCO3(s)
C. H2C2O4(s)
D. Na2CO3(s)
2. A solution contains a mixture of SO42- and S2-. Which of the following cations could be used to remove only the SO42- from the solution by precipitation?
A. K+
B. Sr2+
C. Pb2+
D. Cu2+
3. Consider the following solubility equilibrium: MgCO3(s)⇄ Mg2+(aq) + CO32-(aq)
The addition of which of the following substances would decrease the solubility of MgCO3?
A. H2O
B. NaCl
C. NaOH
D. Na2CO3
4. A student could precipitate silver chloride from a saturated solution of silver chloride by adding
A. Water
B. Sodium iodide
C. Sodium nitrate
D. Sodium chloride
5. The greatest mass of solid SnS will dissolve in 1.0 L of
A. H2O
B. 0.10 M MgS
C. 0.10 M (NH4)2S
D. 0.10 M Sn(NO3)2
6. Magnesium carbonate would be the most soluble in a solution of
A. MgCl2
B. NaNO3
C. Na2CO3
D. Mg(NO3)2
7. Sodium iodide is added to a saturated solution of lead (II) iodide. The net change is
A. [I-] increases and [Pb2+] increases
B. [I-] decreases and [Pb2+] decreases
C. [I-] increases and [Pb2+] decreases
D. [I-] decreases and [Pb2+] increases
8. Consider the following equilibrium: Pbl2(s) + heat ⇄ Pb2+(aq) + 2I-(aq)
Which of the following changes would result in more Pbl2 dissolving?
A. Adding more Pbl2
B. Increasing the pressure
C. Adding some Pb(NO3)2
D. Increasing the temperature
9. Consider the following equilibrium: AgCl(s) ⇄ Ag+(aq) + Cl-(aq)
Sodium chloride is added to a saturated solution of AgCl. The amount of solid AgCl will
A. Increase as the equilibrium shifts to the left
B. Decrease as the equilibrium shifts to the left
C. Increase as the equilibrium shifts to the right
D. Decrease as the equilibrium shifts to the right
10. Consider the following equilibrium: NH4Cl(s) + energy ⇄ NH+4(aq) + Cl-(aq)
Which of the following will increase the solubility of ammonium chloride?
A. Stirring the solution
B. Adding more water
C. Adding more NH4Cl(s)
D. Increasing the temperature
Quiz #8 Titrations
and Max Ion Concentration
1. What is the maximum [Ag+] that can exist in 0.20 M NaBrO3?
A. 1.1 x 10-5 M
B. 5.3 x 10-5 M
C. 2.6 x 10-4 M
D. 7.3 x 10-3 M
2. What is the maximum [Sr2+] that can exist in a solution of 0.10 M Na2SO4?
A. 3.4 x 10-7M
B. 3.4 x 10-6 M
C. 1.7 x 10-6 M
D. 5.8 x 10-4 M
3. A student titrates a 25.00 mL sample of well water with 18.2 mL 0.100 M AgNO3 to completely precipitate the chloride ion. The [Cl-] is
A. 1.82 x 10-3 M
B. 7.28 x 10-2 M
C. 1.37 x 10-1 M
D. 1.50 x 10-1 M
4. What is the maximum concentration of sodium sulphate, Na2SO4, that will dissolve in 1.0 L of 0.10 M Pb(NO3)2 without forming a precipitate?
A. 1.8 x 10-8 M
B. 1.8 x 10-7 M
C. 1.3 x 10-4 M
D. 1.0 x 10-1 M
5. Consider the following equilibrium: AgCl(s) ⇄ Ag+(aq) + Cl-(aq)
When Br-(aq) is added to a saturated solution of AgCl,
A. More AgCl dissolves and its solubility product increases
B. More AgCl precipitates and its solubility product decreases
C. More AgCl dissolves and its solubility product remains constant
D. More AgCl precipitates and its solubility product remains constant
6. In a saturated solution of zinc hydroxide, at 40oC, the [Zn2+] = 1.8 x 10-5 M.
The Ksp of Zn(OH)2 is
A. 5.8 x 10-15
B. 2.3 x 10-14
C. 1.8 x 10-14
D. 6.5 x 10-10
7. What is the [Co2+] and [Cl-] when 0.35 mol of CoCl2 is dissolved in enough water to make 100.0 mL of solution?
A. [Co2+] = 3.5 M and [Cl-] = 3.5 M
B. [Co2+] = 3.5 M and [Cl-] = 7.0 M
C. [Co2+] = 0.35 M and [Cl-] = 0.35 M
D. [Co2+] = 0.35 M and [Cl-] = 0.70 M
8. In which of the following would solid AgCl be most soluble?
A. 1 M HCl
B. 1 M MgCl2
C. 1 M AgNO3
D. 1 M NH4NO3
9. At 25oC, the maximum [Zn2+] that can exist in a 0.250 M Na2S is
A. 5.0 x 10-26
B. 2.0 x 10-25
C. 8.0 x 10-25
D. 4.5 x 10-13
10. The molar solubility of iron (II) sulphide is
A. 3.6 x 10-37
B. 3.0 x 10-19
C. 6.0 x 10-19
D. 7.7 x 10-10
Solubility Web Review
1. Describe the relationship between the rate of dissolving solid and rate of crystallization:
a) A saturated solution and some solid
b) An unsaturated solution and some solid
c) A supersaturated solution and some solid is added
2. Write the equilibrium expression and Ksp equation for Fe2O3.
3. Write the net ionic equation for the reaction between Al(NO3)3 and Na2CO3. Note the difference between this equation and the last one.
4. Mg+2, Sr+2, Ca+2 and Be+2 are possibly in a solution. The solution reacts with Na2SO4 but not NaOH or Na2S. What cations are in the solution?
5. A solution contains SO42- or OH- or both. It reacts with Zn(NO3)2and Sr(NO3)2, What anions are in the solution?
6. Ag2CO3(s) ⇄ 2Ag+ + CO32- Describe the effect on the solubility of Ag2CO3 for each change below:
a) Add Ag2CO3
b) Add
water
c) Add
NaCl
d) Add
Pb(NO3)2
e) Add
Na2CO3
f) Add
AgNO3.
7. If the trial Ksp = 1.7x10-7 and the Ksp = 3.8x10-7 will a precipitate occur?
8. For a saturated solution of Fe(OH)3 the [OH-] is found to be 1.3x10-4 M. Calculate the [Fe+3] and the solubility of the salt in moles/L.
9. Consider the equilibrium that exists in a saturated solution of PbCl2. Write the equilibrium expression. If the equilibrium shifts to the right, what affect does this have on the solubility? Describe how the addition of each of the following will affect the solubility of PbCl2.
a) AgNO3
b) NaCl
c) Na2S
d) H2O
e) NaNO3
f) Pb(NO3)2
10. If the reaction is endothermic, how do the Ksp and the solubility change if the temperature is increased? What is the only way to change the Ksp?
Calculations
1. In a titration 250 mL of a .200 M AgNO3 solution was used to precipitate out all of the Cl- in a 500 mL sample. Calculate [Cl-].
2. In a titration 26.5 mL of .100M Pb(NO3)2 was used to precipitate out all of the I- in a 3.00 mL sample of water. Calculate [I-].
3. Co(OH)2 Solubility = 3.0x10-3 g/L Ksp=?
4. Ag2C2O4 Solubility = 8.3x10-4 M Ksp=?
5. SrF2 Ksp = 2.8 x 10-9 Solubility in (M) = ?
6. Cu(IO3)2 Ksp = 1.4 x 10-7 Solubility (g/L) = ?
7. Calculate the maximum concentration of OH- that can exist in a 0.200M Ca(N03)2 solution. Ksp (Ca(OH)2) = 2.8 x 10-8
8. Calculate the maximum concentration of CO3-2 that can exist in a 0.500 M Fe(NO3)3 solution. Ksp (Fe2(CO3)3) = 2.8 x 10-14
9. Will a precipitate form if 200.0 mL .0020M Mg(NO3)2 is mixed 300.0 mL of 0.0030M NaOH?
10. Will a precipitate form if 25.0mL of .0020M Pb(NO3)2 is mixed with 25.0mL of 0.040M NaBr.
11. 20.0 g of PbCl2 is placed in 2.0 L of water. Some but not all dissolves to form a saturated solution. How many grams do not dissolve?
Solubility Practice
Test # 1
1. Which combination of factors will affect the rate of the following reaction?
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
A. Temperature and surface area only
B. Temperature and concentration only
C. Concentration and surface area only
D. Temperature, concentration, and surface area
2. Consider the following reaction:
2MnO4-(aq) +
5C2O42- + 16H+(aq) → 2Mn2+(aq)
+ 10CO2(g) +
8H2O(l)
The rate of decomposition of the oxalate ion is increased by
A. Adding NaOH
B. Removing CO2
C. Adding a catalyst
D. Decreasing the pressure
3. An equilibrium system shifts left when the temperature is increased. The forward rate is
A. Exothermic and ∆H is positive.
B. Exothermic and ∆H is negative.
C. Endothermic and ∆H is positive.
D. Endothermic and ∆H is negative.
4. The value of the Keq can be changed by
A. adding a catalyst.
B. changing the temperature.
C. changing the reactant concentration.
D. changing the volume of the container
5. Consider the following equilibrium: 2NOCl(g) ⇄ 2NO(g)
+ Cl2(g)
In a 1.0
L container at equilibrium there are 1.0 mol NOCl, 0.70 mol NO and 0.40 mol Cl2.
At constant temperature and volume, 0.10 mol NOCl is added. The concentrations
in the “new” equilibrium in comparison to the concentrations in the “old”
equilibrium are
[NOCl] [NO] [Cl2]
A new = old new = old new = old
B new > old new > old new > old
C new < old new < old new > old
D new < old new > old new > old
6. The equation that represents the equilibrium in a saturated solution of Fe2(SO4)3 is
A. Fe2(SO4)3(s) ⇄ 3Fe2+(aq) + 2SO43-(aq)
B. Fe2(SO4)3(s) ⇄ 2Fe2+(aq) + 3SO43-(aq)
C. Fe2(SO4)3(s)
⇄ 3Fe3+(aq)
+ 2SO42-(aq)
D. Fe2(SO4)3(s) ⇄ 2Fe3+(aq) + 3SO42-(aq)
7. When equal volumes of 0.20 M K2CrO4 and 0.20 M AgNO3 are mixed, a red precipitate is formed. The net ionic equation for this reaction is
A. K+(aq) + NO3-(aq) → KNO3(s)
B. 2Ag+(aq) + CrO42-(aq) → Ag2CrO4(s)
C. K2CrO4(aq) + 2AgNO3(aq) → Ag2CrO4(s) + 2KNO3(aq)
D. 2Ag+(aq) +
CrO4-(aq)
+ 2K+(aq) +
2NO3-(aq)
→ Ag2CrO4(s) +
2KNO3(s)
8. Which of the following compounds could be used to prepare a 0.20 M solution of hydroxide ion?
A. KOH
B. Fe(OH)3
C. Mg(OH)2
D. Zn(OH)2
9. When 250 mL of 0.36 M Sr(OH)2 are added to 750 mL of water, the resulting ion concentrations are
A. [Sr2+] = 0.12 M and [OH-] = 0.12 M
B. [Sr2+] = 0.12 M and [OH-] = 0.24 M
C. [Sr2+] = 0.090 M and [OH-] = 0.090 M
D. [Sr2+] = 0.090 M and [OH-] = 0.180 M
10. When equal volumes of 2.0 M Pb(NO3)2 and 2.0 M KCl are mixed,
A. a precipitate forms because the trial ion product < Ksp
B. a precipitate forms because the trial ion product > Ksp
C. a precipitate does not form because the trial ion product < Ksp
D. a precipitate does not form because the trial ion product > Ksp
11. Consider the following equilibrium: AgCl(s) ⇄ Ag+(aq) + Cl-(aq)
When Br-(aq) is added to a saturated solution of AgCl,
A. more AgCl dissolves and its solubility product increases.
B. more AgCl precipitates and its solubility product decreases.
C. more AgCl dissolves and its solubility product remains constant.
D. more AgCl precipitates and its solubility product remains constant.
12. The molar solubility of iron II sulphide is
A. 3.6 x 10-37 M
B. 3.0 x 10-19 M
C. 6.0 x 10-19 M
D. 7.7 x 10-10 M
13. A solution containing an unknown cation was added to three solutions and the following observations were recorded:
Solution NaI Na2SO4 NaOH
Observation no precipitate precipitate no precipitate
The unknown cation is
A. Pb2+
B. Sr2+
C. Ca2+
D. Ag+
14. If the solubility of Pb(OH)2 is 0.155 g/L, then the concentration of each ion in a saturated solution is
A. [Pb2+] = 0.155 g/L and [OH-] = 0.155 g/L
B. [Pb2+] = 0.155 g/L and [OH-] = 0.103 g/L
C. [Pb2+] = 6.43 x 10-4 M and [OH-] = 1.29 x 10-3 M
D. [Pb2+] = 6.43 x 10-4 M and [OH-] = 6.43 x 10-3 M
15. Which of the following could be used to separate Pb2+ from Ba2+ by precipitation?
A. Na2S
B. NaOH
C. Na2CO3
D. Na2SO4
16. When dissolved in water, which of the following form a molecular solution?
A. HCl(g)
B. NaNO3(s)
C. CH3OH(l)
D. K2SO4(s)
17. Which of the following will be most soluble in water at 25 oC.
A. AgI
B. PbS
C. MgSO4
D. Ba(OH)2
18. At 25 oC, the solubility of Mg(OH)2 is
A. 1.1 x 10-32 M
B. 1.1 x 10-12 M
C. 1.1 x 10-6 M
D. 1.1 x 10-4 M
19. At 25 oC, the solubility of an unknown compound is 7.1 x 10-5 M. The compound is
A. CuI
B. AgI
C. CaCO3
D. CaSO4
20. When solid AgBr is added to a saturated solution of AgBr, the reaction rates can be described as:
Rate of Dissolving Rate of Crystalizing
A. increasing increasing
B. increasing decreasing
C. decreasing increasing
D. increasing no change
21. The solubility of PbS is 2.9 x 10-14 M. What is the value of the Ksp.
A. 8.4 x 10-28
B. 2.9 x 10-14
C. 5.8 x 10-14
D. 1.7 x 10-7
22. Which of the following causes a precipitate to form when Sr2+(aq) is added but not when Zn2+(aq) is added?
A. S2-
B. Cl-
C. SO42-
D. CO32-
23. A 3.0 L solution of NiCl2 is found to have a chloride concentration of 0.60 M. The concentration of nickel II ions is
A. 0.30 M
B. 0.60 M
C. 0.90 M
D. 1.2 M
24. When equal volumes of 0.20 M K2CO3 and 0.2 M Na3PO4 are mixed,
A. no precipitate will form
B. a precipitate of K3PO4 will form
C. a precipitate of Na2CO3 will form
D. a precipitate of K3PO4 and Na2CO3 will form
25. A solution of AgNO3 is slowly added to a mixture containing 0.10 M I-, Cl-, Br-, and IO3-. The precipitate which forms first is
A. AgI
B. AgCl
C. AgBr
D. AgIO3
26. Which of the following units can be used to represent solubility?
A. g
B. mol
C. mol/L
D. mL/s
27. Consider the following equilibrium: CaCO3(s) ⇄ Ca2+(aq) + CO32-(aq)
Which of the following reagents when added to the equilibrium system, would cause more CaCO3 to dissolve?
A. KNO3(s)
B. CaCO3(s)
C. H2C2O4(s)
D. Na2CO3(s)
28. Which of the following could be used to precipitate both Mg2+ and Ca2+ from hard water?
A. lithium sulphate
B. sodium phosphate
C. potassium sulphide
D. ammonium chloride
29. What is the maximum [Ag+] that can exist in 0.20 M NaBrO3?
A. 1.1 x 10-5 M
B. 5.3 x 10-5 M
C. 2.6 x 10-4 M
D. 7.3 x 10-3 M
30. Which of the following ions could be used to separate Cl-(aq) from SO42-(aq) by precipitation?
A. Ag+
B. Ca2+
C. NH4+
D. Pb2+
31. The Ksp expression for a saturated solution Ca3(PO4)2 is
A. Ksp = [Ca2+][PO43-]
B. Ksp = [Ca2+]3[PO43-]2
C. Ksp = [3Ca2+][2PO43-]
D. Ksp = [3Ca2+][2PO43-]
32. When Ca(OH)2 attains solubility equilibrium, the
A. solution will be saturated
B. pH will be less than 7
C. trial Ksp is less than the Ksp
D. concentrations of the ions are equal
33. Which of the following describes the changes in ion concentrations when 1.0 g of solid ZnS is added to a saturated solution of ZnS?
[Zn2+] [S2-]
A. increases decreases
B. decreases decreases
C. increases increases
D. remains constant remains constant
34. The net ionic equation for the reaction between Sr(OH)2 and H2SO4 is
A. H+ + OH- → H2O
B. Sr2+ + SO42- → SrSO4
C. Sr(OH)2 + H2SO4 → SrSO4 + 2H2O
D. Sr2+ + 2OH- + 2H+ + SO42- → SrSO4 + 2H2O
35. The relationship between the solubility and the size of the Ksp is
A. there is no relationship
B. the smaller the Ksp the greater the solubility
C. the greater the Ksp the greater the solubility
D. the solubility is always the square root of the Ksp
36. Which of the following compounds will form a saturated solution with the greatest concentration of Ag+?
A. AgI
B. AgBr
C. AgIO3
D. AgBrO3
37. When equal volumes of 0.20 M CuSO4 and 0.20 M Li2S are combined, the complete ionic equation is
A. Cu2+(aq) + S2-(aq) → CuS(s)
B. CuSO4(aq) + Li2S(aq) → CuS(s) + Li2SO4(aq)
C. Cu2+(aq) +
SO42-(aq)
+ 2Li+(aq) +
S2-(aq) → CuS(s) + Li2SO4(aq)
D. Cu2+(aq) + SO42-(aq) + 2Li+(aq) + S2-(aq) → CuS(s) + 2Li+(aq) + SO42-(aq)
38. Which of the following would have the [Fe3+] = 0.020 M?
A. 0.40 L of 0.050 M Fe(NO3)3
B. 0.80 L of 0.020 M Fe2(SO4)3
C. 0.50 L of 0.040 M FeC6H5O7
D. 0.50 L of 0.010 M Fe2(C2O4)3
39. A solution contains both Ag+ and Mg2+ ions. During selective precipitation, these ions are removed one at a time by adding
A. I- followed by OH-
B. OH-
followed by S2-
C. SO42- followed by
Cl-
D. NO3- followed by PO43-
40. The solubility of an AB2 type salt is 2.3 x 10-6 M. The salt is
A. PbBr2
B. Fe(OH)2
C. Cu(IO3)2
D. Mg(OH)2
1. A saturated solution of BaSO4 is given to patients needing digestive tract x-rays.
a) Write an equation that represents the solubility equilibrium
b) Calculate the [Ba2+] present in the saturated solution.
2. Will a precipitate form when 90.0 mL of 1.00 x 10-2 M Cu(NO3)2 and 10.0 mL of
1.00 x 10-2 M NaIO3 are mixed? Explain using appropriate calculations.
3. What is the maximum [CO32-] that can exist in a 1.3 x 10-4 M AgNO3 solution?
4. The following data was collected when a 25.00 mL sample of water containing chloride ion was titrated using 0.100 M AgNO3 to completely precipitate the chloride ion.
Initial volume of AgNO3 18.30 mL
Final volume of AgNO3 27.22 mL
a) Write the net ionic reaction for the precipitation reaction.
c) Calculate the [Cl-].
5. In an experiment to determine the solubility of BaF2, 500.0 mL of the saturated solution was heated in an evaporating dish to remove the water. The evaporating dish and the residue were then heated two more times to ensure all the water was removed.
Volume of the saturated solution of BaF2 500.0 mL
Mass of the evaporating dish 72.540 g
Mass of the evaporating dish and BaF2 after the first heating 73.500 g
Mass of the evaporating dish and BaF2 after the second heating 72.855 g
Mass of the evaporating dish and BaF2 after the third heating 72.855 g
Using the above data, calculate the Ksp for BaF2
Chemistry 12 Solubility Test # 2
1. Consider the following experiment:
1.0 mL 0.20 M Ag+ + an unknown solution → precipitate
1.0 mL 020 M Sr2+ + an unknown solution → no precipitate
The unknown solution could contain
A 0.20 M OH-
B 0.20 M NO3-
C 0.20 M PO43-
D 0.20 M SO42-
2. A compound has a solubility of 7.1 x 10-5 M at 25 oC. The compound is
A CuS
B AgBr
C CaCO3
D CaSO4
3. A saturated solution of NaCl contains 36.5 g of solute in 0.100 L of solution. The solubility of the compound is
A 0.062 M
B 1.60 M
C 3.65 M
D 6.24 M
4. Calculate the [Li+] in 200.0 mL of 1.5 M Li2SO4.
A 0.30 M
B 0.60 M
C 1.5 M
D 3.0 M
5. The Ksp expression for a saturated solution of Mg(OH)2 is
A Ksp = [Mg2+][OH-]2
[Mg(OH)2]
B Ksp = [Mg2+][OH-]2
C Ksp = [Mg2+][OH-]
D Ksp = [Mg2+][2OH-]2
6. Consider the following saturated solution solutions
CuSO4 BaSO4 CaSO4
The order of cation concentration, from highest to lowest, is
A [Ba2+] > [Ca2+] > [Cu2+]
B [Ca2+] > [Cu2+] > [Ba2+]
C [Cu2+] > [Ca2+] > [Ba2+]
D [Cu2+] > [Ba2+] > [Ca2+]
7. When 1.0 x 10-3 moles of CuCl2(s) are added to 1.0 L of 1.0 x 10-3 M IO3-, the
A Trial Ksp > Ksp and a precipitate forms
B Trial Ksp < Ksp and a precipitate forms
C Trial Ksp > Ksp and no precipitate forms
D Trial Ksp < Ksp and no precipitate forms
8. The solubility of CdS = 2.8 x 10-14. The value of the Ksp is
A 7.8 x 10-28
B 2.8
x 10-14
C 5.6
x 10-14
D 1.7 x 10-7
9. The ion concentrations in 0.25 M Al2(SO4)3 are
[Al3+] [SO42-]
A 0.25 M 0.25 M
B 0.50 M 0.75 M
C 0.75 M 0.50 M
D 0.10 M 0.15 M
10. Which of the following will not produce a precipitate when equal volumes of 0.20 M solutions are combined?
A KOH and CaCl2
B Zn(NO3)2 and K3PO4
C Sr(OH)2 and (NH4)2S
D Na2SO4 and Pb(NO3)2
11. Consider the following equilibrium: Mg(OH)2(s) ⇄ Mg2+(aq) + 2OH-(aq)
A compound that can be added to cause a shift to the right is
A NaOH
B HCl
C Sr(OH)2
D Mg(OH)2
12. If the trial ion product for AgBrO3 is calculated to be 1.0 x 10-7, then
A a precipitate forms because the trial ion product > Ksp
B a precipitate forms because the trial ion product < Ksp
C no a precipitate forms because the trial ion product > Ksp
D no a precipitate forms because the trial ion product < Ksp
13. Which of the following will dissolve in water to produce a molecular solution?
A CaCl2
B NaOH
C CH3OH
D Sr(OH)2
14. In a solubility equilibrium, the
A rate of dissolving equals the rate of crystallization
B neither dissolving or crystallization occurs
C concentration of solute and solvent are equal
D mass of dissolved solute is greater than the mass of the solution
15. The maximum [SO42-] that can exist in 1.0 x 10-3 M Ca(NO3)2 without a precipitate forming is
A 7.1 x 10-5 M
B 1.0 x 10-3 M
C 8.4 x 10-3 M
D 7.1 x 10-2 M
16. When equal volumes of 0.20 M CuSO4(aq) and 020 M Li2S(aq) are combined, the complete ionic equation is
A Cu2+(aq) + S2-(aq) → CuS(s)
B CuSO4(aq) + Li2S(aq) → CuS(s) + Li2SO4(s)
C Cu2+(aq) + SO42-(aq) + 2Li+(aq) + S2-(aq) → Li2SO4(aq) + CuS(s)
D Cu2+(aq) + SO42-(aq) + 2Li(aq) + S2-(aq) → CuS(s) + 2Li+(aq) + SO42-(aq)
17. Consider the solubility equilibrium: CaCO3(aq) ⇄ Ca2+(aq) + CO32-(aq)
An additional piece of solid CaCO3 is added to the equilibrium above. The rate of dissolving and the rate of crystallization have
Rate of Dissolving Rate of crystallization
A increases increases
B increases not changed
C not changed increased
D not changed not changed
18. At 25 oC, which of the following compounds would dissolve to form a saturated solution with the greatest [Pb2+]?
A PbI2
B PbCl2
C PbBr2
D Pb(IO3)2
19. Consider the following anions:
I 10.0 mL of 0.20 M Cl-
II 10.0 mL of 0.20 M OH-
III 10.0 mL of 0.20 M SO32-
When 10.0 mL of 0.20 M Pb(NO3)2 are added to each of the above, precipitates form in
A I and II only
B I and III only
C II and III only
D I, II, and III
20. Which of the following units could be used to describe solubility?
A g/s
B g/L
C M/L
D mol/s
21. The solubility of SnS is 3.2 x 10-3 M. The value of the Ksp is
A 1.0
x 10-5
B 3.2
x 10-3
C 6.4
x 10-3
D 5.7 x 10-2
22. Silver chloride, AgCl, would be least soluble in
A 1.0 M HCl
B 1.0 M NaNO3
C 1.0 M ZnCl2
D 1.0 M AgNO3
23. The solubility of SrF2 is
A 4.3 x 10-9
B 6.6 x 10-5
C 1.0 x 10-3
D 1.6
x 10-
24. The Ksp expression for a saturated solution of Ag2CO3 is
A Ksp = [Ag2+][CO32-]
B Ksp = [Ag+]2[CO32-]
C Ksp = [2Ag+][CO32-]
D Ksp = [2Ag+]2[CO32-]
25. How many moles of solute are dissolved in 200.0 mL of a saturated solution of FeS?
A 1.2 x 10-19
B 6.0 x 10-19
C 1.5 x 10-10
D 7.7 x 10-10
26. A solution contains both Ag+ and Mg2+ ions. During selective precipitation, these ions are removed one at a time by adding
A I- followed by OH-
B OH- followed by S2-
C SO42- followed by
Cl-
D NO3- followed by
PO43-
27. Which of the following does not define solubility?
A the concentration of solute in a saturated solution
B the moles of solute dissolved in a given amount of solution
C the maximum mass of solute that can dissolve in a given amount of solution
D the minimum amount of solute required to produce one litre of saturated solution
28. The ion concentrations in 0.25 M Al2(SO4)3 are
[Al3+] [SO42-]
A 0.25 M 0.25 M
B 0.50 M 0.75 M
C 0.75 M 0.50 M
D 0.10 M 0.15 M
29. Which of the following will not produce a precipitate when equal volumes of 0.20 M solutions are combined?
A KOH and SrCl2
B Zn(OH)2 and K3PO4
C Zn(OH)2 and (NH4)2S
D Na2SO4 and Pb(NO3)2
30. What is observed when H2SO4 is added to a saturated solution of CaSO4?
A CaSO4(s) dissolves
B the [Ca2+] increases
C bubbles of H2 are given off
D additional CaSO4 precipitates
31. The solubility of CdS is 2.8 x 10-14 M. The value of the Ksp is
A 7.8
x 10-28
B 2.8
x 10-14
C 5.6
x 10-14
D 1.7 x 10-7
32. Consider the following solutions: 0.10 M Cl- 0.10 M Br-
0.10 M IO3- 0.10 M BrO3-
Equal moles of AgNO3 are added to each solution. It is observed that a precipitate forms in all but one solution. Which solution does not form a precipitate?
A Cl-
B Br-
C IO3-
D BrO3-
33. Consider the following equilibrium: 2O3(g) ⇄ 3O2(g) Keq = 65
Initially, 0.10 mole O3 and 0.10 mole of O2 are placed in a 1.0 L container. Which of the following describes the changes in concentrations as the reaction proceeds to equilibrium?
[O3] [O2]
A decreases decreases
B decreases increases
C increases decreases
D increases increases
34. Consider the following potential energy diagram for the reversible reaction.
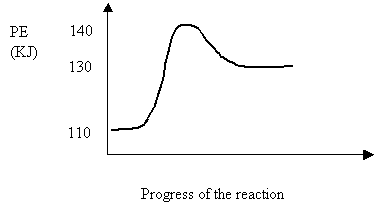
Ea
reverse (kJ) ΔH
(kJ)
A 10 -20
B 10 -30
C 30 +10
D 20 +30
35. Increasing the temperature of a reaction increases the rate by
I increasing frequency of collision
II increasing the kinetic energy of collision
III decreasing the potential energy of collision
A I only
B I and II only
C II and III only
D I, II, and III
36. What is the Keq expression for the following equilibrium?
Fe (s) + 4H2O(g) ⇄ Fe3O4(s) + 4H2(g)
A Keq = [H2]4
B Keq
= [H2]
[H2O]
C Keq = [H2]4
[H2O]4
D Keq
= [Fe3O4][H2]4
[Fe]3[H2O]4
Subjective
1. Write the net ionic equation representing the reaction that occurs when 50.0 mL of
0.20 M ZnSO4 and 50.0 mL 0.20 M BaS are combined.
2. A 100.0 mL sample of 0.600M Ca(NO3)2 is diluted by adding 400.0 mL of water. Calculate the concentrations of all of the ions.
3. When 1.00 L of CaF2 was evaporated to dryness, 2.66 x 10-2 g of residue was formed. Calculate the Ksp.
4. A maximum of 0.60 g Pb(NO3)2 can be added to 1.5 L of 0.100 M NaBr(aq) without forming a precipitate. Calculate the ksp of PbBr2.
5. Consider the following solutions at 25 oC
Saturated AgCl(aq) Saturated Ag2CO3(aq)
Using calculations, identify the solution with the greater [Ag+].