Equilibrium Worksheet Questions
Power Point Lesson Notes- double
click on the lesson number.
![]()
Worksheets Quiz
1. Approaching Equilibrium WS 1
Q1
2. LeChatelier's Principle-1 WS 2
3. LeChatelier's Principle-2 WS 3 &
4 Q2
4. LeChatelier's-3 & Start Lab WS 5
5. Lab
Lechatelier's Questions 1-10 Conclusion
7. Equilibrium Constants WS 8 Q4
9. K-trial & Size Keq WS
11
Q5
10. Entropy & Enthalpy WS
12 Q6
11. Review
Web Review Practice Test 1
12. Review Practice
Test 2 Quizmebc
Read Hebdon Unit 2
Worksheet #1 Approaching Equilibrium
Read
unit II your textbook. Answer all of the questions. Do not start the questions
until you have completed the reading. Be prepared to discuss your answers next
period.
1. What are the conditions necessary for equilibrium?
Must have a closed
system.
Must have a constant temperature.
Ea must be low enough to allow a reaction.
2. What is a forward reaction versus a reverse reaction?
In a forward reaction, the reactants collide
to produce products and it goes from left to right.
In a reverse reaction, the products collide
to produce reactants and it goes form right to left.
3. Why does the forward reaction
rate decrease as equilibrium is approached?
As the reaction
goes to the right, the reaction concentration decreases and therefore, there are
less reactant collisions causing the forward rate to decrease.
4. What are the characteristics of equilibrium?
Forward rate is equal to the reverse rate.
The concentration of reactants and products
are constant.(not equal)
Macroscopic properties are constant (color,
mass, density, pressure, concentrations).
5. Define equilibrium.
Equilibrium occurs when:
Forward rate is equal to the reverse rate.
The concentration of reactants and products
are constant.(not equal)
Macroscopic properties are constant. (color,
mass, density, pressure, concentrations)
6. Define the word dynamic and explain its relevance
to the concept of equilibrium.
The word dynamic means that forward and
reverse continue to occur.
7. Why does the reverse reaction rate increase as equilibrium
is approached?
The reverse reaction rate increases as
equilibrium is approached because as the reaction goes from left to right,
the concentrations of the products increases,
therefore there are more product collisions causing the reverse reaction rate
to increase.
As a reaction is approaching
equilibrium describe how the following change. Explain what causes each change.
8. Reactant concentration. As the reaction goes to the right,
the reaction concentration decreases.
9. Products concentration. As the reaction goes from left to right, the concentration
of the products increases.
10. Forward reaction rate. The reaction concentration decreases and therefore, there
are less reactant collisions causing the forward rate to decreases.
11. Reverse reaction rate. The concentrations of the products increases, therefore there
are more product collisions causing the reverse reaction rate to increase.
12. What is equal at equilibrium? The forward and reverse rates are equal.
13. What is constant at equilibrium? The reactant and product concentrations and the macroscopic
properties are constant.
14. Sketch each graph to show how concentrations change
as equilibrium is approached
[reactant]
[product] Overall
Rate
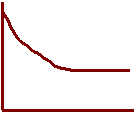

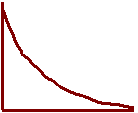
15. Label each graph with the correct description.
· The forward and reverse rates
as equilibrium is approached
· The overall rate as equilibrium
is approached
· The reactant and product concentrations
as equilibrium is approached (two graphs)
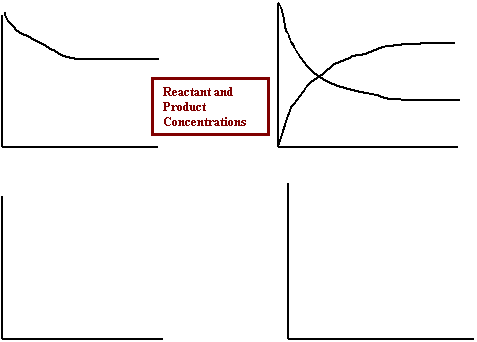
![]()
![]()
16. Draw a PE Diagram for the
reaction if PE of the reactants is 100 KJ/mole N2O4 and
Ea = 110 KJ/mole N2O4.
N2O4 (g) <----->
2 N02 (g) DH= +58KJ
(colorless)
(brown)
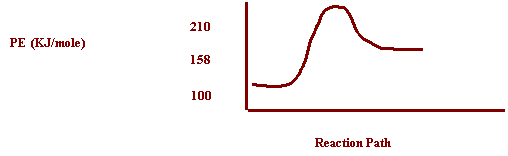
If
a catalyst was added to the reaction, what would happen to the PE Diagram, the
forward rate, and the reverse rate?
PE
Diagram The
activation energy would decrease
Forward
rate Increase
Reverse
rate Increase
One
mole of very cold, colorless N2O4 (g) is placed into a 1.0L
glass container of room temperature. The reaction:
N2O4 (g) ⇋ 2 N02 (g) DH= +58KJ
(colorless)
(brown)
proceeds to equilibrium. The
concentration of each gas is measured as a function of time.
Time (s)
0 5 10 15
20 25
[N2O4]
(M) 1.0 0.83 0.81 0.80 0.80 0.80
[N02] (M) 0.0 0.34 0.38 0.40 0.40 0.40
17. Plot concentration of N2O4
and N02 against time on the same graph below.
 1.0 -
1.0 -
0.9 -
0.8 -
0.7 -
0.6 -
0.5 -
0.4 -
0.3 -
0.2 -
0.1 -
0.0 -
0 5
10 15 20 25
30 35
TIME (s)
18. After what time interval
has equilibrium been established? 15 seconds
19.
Describe the change in the appearance of the container over 25 seconds (describe
the colour change and when it becomes constant).
The
container will gradually increase the intensity of brown and then remain constant
after 15 seconds.
20. Calculate
the rate of N2O4 consumption in (M/s) over the first 5s
period and then the second 5s period.
0-5 sec.
rate = 1.0 – 0.83 M = 0.034 M / s
5.0 sec
5-10 sec.
rate = 0.83 – 0.81 M = 0.004 M / s
5.0 sec
Why is the rate greater over the first five minutes compared to
the second five minutes (think in terms of reactant and product concentrations?
The reactant
concentration has decreased and the product concentration increased.
The forward rate
has decreased and the reverse rate increased and because of this the overall net
rate has decreased.
21. Calculate the rate of N02
production in (M/s) over the first 5s period and then the second 5s period.
0-5 sec.
rate = 0.34 – 0.00 M = 0.068 M / s
5.0 sec
5-10 sec.
rate = 0.38 – 0.34 M = 0.008 M / s
5.0 sec
How does the rate of formation of N02 compare to the
rate of consumption of N2O4? Remember, if you measure the
reactants or products, it is still the overall rate.
It is twice as great because of the stoichiometric relationship.
2moles NO2
1mole N2O4
22. What are the equilibrium
concentrations of N2O4 and N02?
[N2O4]= 0.80 M Are they equal?
No!
[N02] = 0.40 M
23. Is the reaction over, when
equilibrium has been achieved? If not, explain.
No it is not. Although the concentrations
are constant, the forward and reverse reactions continue forever.
24. What are
the necessary conditions to establish equilibrium?
Must have
a closed system.
Must have a constant
temperature.
Ea must be low enough to allow a reaction.
25. What are the characteristics
of an equilibrium?
Forward rate is
equal to the reverse rate.
The concentration
of reactants and products are constant.(not equal)
Macroscopic properties
are constant. (color, mass, density, pressure, concentrations)
Worksheet #2 LeChatelier’s
Principle
Describe
the changes that occur after each stress is applied to the equilibrium.
N2 (g) + 3H2 (g) ⇋ 2NH3(g) +
92 KJ
Shifts
Shifts to the
Stress [N2] [H2] [NH3] Right or Left Reactants or Product
1.
[N2] is increased increases decreases
increases
right products
2.
[H2] is increased decreases increases
increases
right products
3.
[NH3] is increased increases increases
increases
left
reactants
4.
Temp is increased increases increases
decreases
left reactants
5.
[N2] is decreased decreases increases
decreases
left reactants
6.
[H2] is decreased increases decreases
decreases left
reactants
7.
[NH3] is decreased decreases decreases
decreases right
products
8.
Temp is decreased decreases decreases
increases right products
9.
A catalyst is added nochange nochange
nochange
nochange nochange
N2O4
(g) ⇋ 2NO2(g)
DH = +
92 KJ
Shifts
Shifts to Favor the
Stress
[N2O4] [NO2]
Right or Left
Reactants or Products
1.
[N2O4] is increased
increases increases right
products
2.
[NO2] is increased
increases increases left
reactants
3.
Temp is increased decreases increases right
products
4.
[N2O4] is decreased
decreases decreases left
reactants
5.
[H2] is decreased
nochange nochange nochange
nochange
6.
[NO2] is decreased
decreases decreases right
products
7.
Temp is decreased increases decreases left
reactants
4HCl
(g) + O2
(g) ⇋ 2H2O(g) + 2Cl2 (g) +
98 KJ
Shifts Shifts to Favour the
Stress [O2] [H2O] [HCl] Right or Left Reactants or Products
1.
[HCl] is increased decreases increases increases right products
2.
[H2O] is increased increases increases increases left reactants
3.
[O2] is increased increases increases decreases right products
4.
Temp is increased increases decreases increases
left
reactants
5.
[H2O] is decreased decreases decreases decreases
right products
6.
[HCl] is decreased increases decreases decreases
left
reactants
7.
[O2] is decreased decreases decreases increases
left
reactants
8.
Temp is decreased decreases increases decreases right products
9.
A catalyst is added nochange nochange nochange
nochange nochange
CaCO3 (s) + 170
KJ ⇋ CaO (s) +
CO2 (g)
Note : Adding solids or liquids and removing solids
or liquids does not shift the equilibrium. This is because you cannot change the
concentration of a pure liquid or solid as they are 100% pure. It is only a concentration
change that will change the # of collisions and hence shift the equilibrium.
Shifts Shifts to Favor the
Stress [CO2] Right or Left Reactants or Products
1.
CaCO3 is added nochanges
nochanges nochanges
2.
CaO is added nochanges
nochanges nochanges
3.
CO2 is added increases
left reactants
4.
Temp is decreased decreases
left reactants
5.
A catalyst is added nochanges
nochanges nochanges
6.
[CO2] is decreased decreases
right products
7.
Temp is increased increases
right products
8.
CaO is removed nochanges
nochanges nochanges
Worksheet #3 Applying Le Châtelier's Principle
The
oxidation of ammonia is a reversible exothermic reaction that proceeds as follows:
4 NH3 (g) + 5 O2
(g) ⇋ 4 NO (g) + 6 H2O (g)
Le Châtelier’s Principle allows us to predict the changes that occur in an equilibrium reaction
to compensate for any stress that is placed upon the system. For each situation described in the table,
indicate an increase or decrease in overall concentration from before to after a new equilibrium has
been established.
Component Stress Equilibrium Concentrations
[NH3] [O2]
[NO] [H2O]
NH3 addition increases decreases increases increases
removal decreases increases decreases decreases
removal increases decreases decreases decreases
removal decreases decreases decreases increases
H2O
addition increases increases decreases increases
removal decreases decreases increases decreases
Increase in temperature: increases increases decreases decreases
Decrease in temperature: decreases decreases increases increases
Increase Presssure: increases increases increases increases
Decrease in pressure: decreases decreases decreases decreases
We increased the volume- all concentrations go down
Addition of a catalyst: nochange nochange nochange nochange
Worksheet #4 Le Chatelier’s Principle
State the direction in which each of the following equilibrium systems would be shifted upon the application
of the following stress listed beside the equation.
1. 2
SO2 (g) + O2 (g) ⇋ 2 SO3 (g)
+ energy decrease temperature right
2. C
(s) + CO2 (g) + energy ⇋ 2 CO (g) increase temperature right
3. N2O4
(g) ⇋ 2 NO2 (g)
increase total pressure left
4. CO
(g) + H2O (g)
⇋ CO2 (g) + H2 (g) decrease total pressure nochange
5. 2
NOBr (g) ⇋ 2 NO (g) + Br2 (g) decrease total pressure right
6. 3
Fe (s) + 4 H2O (g)
⇋ Fe3O4 (s) + 4 H2 (g) add Fe(s) nochanges
7. 2SO2
(g) + O2 (g) ⇋ 2 SO3 (g)
add catalyst nochanges
8. CaCO3
(s) ⇋ CaO (s) + CO2 (g) remove CO2 (g) right
9. N2
(g) + 3 H2 (g)
⇋ 2 NH3 (g)
increase [He (g)] no change
Consider the following equilibrium
system:
3 H2 (g) + N2 (g)
⇋ 2 NH3 (g) + Heat.
State what effect each of the following will have on this system:
10. More
N2 is added to the system
right
11. Some
NH3 is removed from the system right
12. The
temperature is increased
left
13. The
volume of the vessel is increased left
14. A
catalyst was added
nochange
15. An inert gas was added nochange
16. If a catalyst was added to the above reaction and a new equilibrium was established.
Compare to the original system, the rates of the forward and reverse reactions of the new equilibrium.
Forward Rate has increases Reverse Rate has increases
17. If the temperature was increased in the above reaction and a new equilibrium was established.
Compare to the original system, the rates of the forward and reverse reactions of the new equilibrium.
Forward Rate has increases Reverse Rate has increases
18.
If the volume of the container was
increased in the above reaction and a new equilibrium
was established. Compare to the original system, the rates of the forward and reverse reactions
of the new equilibrium.
Forward Rate has decreases Reverse Rate has decreases .
Consider the following equilibrium system
H2 (g) + I2 (g) ⇋ 2 HI (g)
State what effect each of the following will have on this
system in terms of shifting.
19. The volume of the vessel is increased nochange
20. The pressure is increased nochange
21. A
catalyst is added nochange
Consider the following equilibrium
system:
3 Fe (s) + 4 H2O (g) <------> Fe3O4 (s) + 4 H2 (g)
State what effect each of the following will have on this
system in terms of shifting.
21. The volume of the vessel is decreased nochange
22. The pressure is decreased nochange
23. More Fe is added to the system nochange
24. Some Fe3O4 is removed
from the system nochange
25. A catalyst is added to the system nochange
Consider the following equilibrium:
2NO (g) + Br2 (g) + energy <------> 2NOBr (g)
State what affect each of the following will have on this
system in terms of shifting.
26. The volume of the vessel is increased left
27. The pressure is decreased left
28. More Br2 is added to the system
right
29. Some NO is removed from the system left
30. A catalyst is added to the system nochange
Consider the following equilibrium:
Some CO was added to the system and
a new equilibrium was established.
2CO (g) + O2 (g)
<------> 2CO2 (g) + energy
31. Compare
to the original system, the rates of the forward and reverse reactions of the
new equilibrium.
Forward
Rate has increases Reverse Rate has
increases
32.
Compared to the original concentrations,
after the shift, have the new concentrations increased or decreased?
[CO]
increases
[O2] decreases [CO2] increases
33. Did
the equilibrium shift favour the formation of reactants or products? products
A catalyst was added to the system
at constant volume and a new equilibrium was established.
2CO
(g) + O2 (g) ⇋ 2CO2 (g) + energy
34. Compare
to the original system, the rates of the forward and reverse reactions of the
new equilibrium.
Forward
Rate has increases Reverse Rate has increases
35. Compared
to the original concentrations, after the shift, have the new concentrations increased
or decreased?
[CO] no change [O2] no change
[CO2] no
change
36. Did
the equilibrium shift favour the formation of reactants or products? Neither
The volume of the container was decreased
and a new equilibrium was established.
2CO
(g) + O2 (g) ⇋ 2CO2 (g) + energy
37. Compare
to the original system, the rates of the forward and reverse reactions of the
new equilibrium.
Forward
Rate has increased Reverse Rate has increased
38. Compared
to the original concentrations, after the shift, have the new concentrations increased
or decreased?
[CO] increased [O2]
increased
[CO2] increased
39. Did
the equilibrium shift favor the formation of reactants or products? Products
Worksheet #5 Applying Le Châtelier's
Principle
1. The chromate and dichromate ions set up an equilibrium as follows:
energy +
2 CrO4 2-(aq) + 2 H+(aq) ⇋ Cr2O7 2-(aq) + H2O
(l)
yellow
orange
Describe how the
above equilibrium will shift after each stress below:
shift
color change
Increase in [H+] right
Orange
Increase in [CrO4 2-] right
Increase in [Cr2O7
2-] left
Decrease in [H+] left
Yellow
Decrease
in [CrO42-]
left
Increase in temperature right Orange
Decrease intemperature left
Yellow
Add HCl (aq) right
Orange
Add NaOH left Yellow
OH- reacts with H+ and lowers [H+]
causing the reaction to shift left.
2. The copper (II) ion andcopper (II) hydroxide complex exist in
equilibrium as follows:
Cu(OH)2 (aq)
+4 H2O (l) ⇋ Cu(H2O)4 2+(aq) +2 OH-(aq)
+ 215 kJ
violet
light blue
Describe how the
above equilibrium will shift after each stressbelow:
shift
colorchange
Increase in [Cu(H2O)4
2+]
left
Add NaOH left
Violet
Increase in [Cu(OH)2] right
Decrease in [Cu(H2O)4
2+]
right
Decrease
in[Cu(OH)2]
left
Increase temperature left
Violet
Decrease temperature right
Light Blue
AddKCl
(aq)
no change nochange
AddHCl
(aq)
right Light Blue
3. Consider the equilibriumthat follows:
4 HCl
(g)+ 2 O2 (g) ⇋ 2 H2O (l) +
2 Cl2 (g) + 98
kJ
(yellow)
Describe how the above equilibrium will shift after each
stress below:
shift
color change
Increase in temperature left
clear
Increase [HCl] right
yellow
Decrease in [Cl2] right
Decrease temperature right
yellow
Add Ne at constant volume No Change
4.
Consider the equilibrium that follows:
Cu+
(aq) + Cl-(aq) ⇋ CuCl (s) ΔH = +
98 kJ
(green)
Describe how the above equilibrium will shift after each stress
below:
Cu+ is green
shift color change
Increase in temperature right less green
Increase [HCl]
right
less green
Add NaCl
right
less green
Decrease temperature
left
green
Add
NaOH (aq)
left
clear
(reacts
with HCl)
(check your solubility table for a
possible reaction)
Add
CuCl(s)
no change
no change
Add
AgNO3 (aq)
left
green
(check your solubility table for a
possible reaction)
Add
CuNO3 (aq)
right because it contains the Cu+ ion.
Add
Cu(NO3)2 (aq)
no change because the Cu2+ ion is a spectator.
Worksheet #6 Graphing and LeChatelier’s Principle
Consider the following equilibrium system.
I2(g)
+ Cl2(g) ⇋ 2 ICl (g) + energy
Label the graph that best represents each of the following
stresses and shift.
· adding I2(g)
· increasing the temperature
· decreasing the pressure
· Increasing the temperature
removing
Cl2(g)
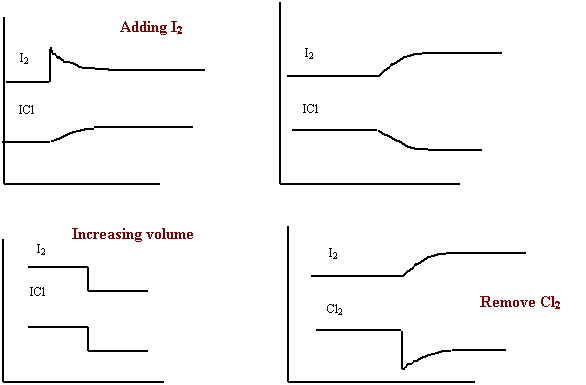
1. N2O4(g) + 59
KJ ⇋ 2 NO2(g)
Describe
four ways of increasing the yield of for the reaction above.
Increase the temperature
Increase the concentration of (N2O4)
Decrease the pressure
Decrease the concentration of (NO2)
Describe
three ways to increase the rate of the above reaction.
Increase the temperature
Increase the concentration of (N2O4)
Add a catalyst
Increase the pressure
2. 2SO3(g) ⇋ 2SO2(g) +
O2(g) + 215
KJ
Describe
four ways of increasing the yield of for the reaction above.
Decrease the temperature
Increase the concentration of SO3
Decrease the pressure
Decrease the concentration of SO2
Decrease the concentration of O2
Describe
three ways to increase the rate of the above reaction.
Increase the temperature
Increase the concentration
of (SO3)
Increase the pressure
3. H2O(g)
® H2O(l) DH = -150 KJ
Describe
three ways of increasing the yield of for the reaction above.
Decrease the temperature
Increase the concentration of H2O
Increase the pressure
Describe
four ways to increase the rate of the above reaction.
Increase the temperature
Increase the concentration of H2O
Add a catalyst
Increase the pressure
4. In
the Haber reaction: 3H2(g) + N2(g)
⇌ 2NH3(g) + energy
Explain
why each condition is used in the process to make ammonia.
A
High pressure of 50 MP
High yield shifts to right
The
presence of Fe2O3
A catalyst increases the rate
Condensing
NH3 to a liquid
Removing the products shifts to the right increasing the
yield
A relatively high temperature 500 oC Even
though the yield is lowered the rate is increased
Worksheet #8
Equilibrium Calculations
Solve each problem and show all of your work.
1. SO3(g) + H2O(g)
⇄ H2SO4(l) Do not count the liquid!!
At equilibrium [SO3] = 0.400M
[H2O] = 0.480M
[H2SO4]
= 0.600M
Calculate the value of the equilibrium
constant.
Keq = 1
[SO3]
[H2O]
Keq
=
1
[0.400] [0.480]
Keq = 5.21
2. At equilibrium at 100oC, a 2.0L flask contains:
0.075 mol of PCl5 0.050 mol of H2O
0.750 mol 0f HCl 0.500 mol of POCl3
Calculate the Keq for the reaction:
PCl5 (s) + H2O (g) ⇄ 2HCl (g) + POCl3 (g)
Keq = 1.4
3. Keq= 798 at 25oC for the reaction: 2SO2
(g) + O2 (g) ⇄ 2SO3 (g).
In a particular mixture at equilibrium,
[SO2]= 4.20 M and [SO3]=11.0M. Calculate the equilibrium
[O2] in this mixture at 25oC.
[O2]
= 0.00860M
4. Consider the following equilibrium:
2SO2 (g) + O2 (g) ⇄ 2SO3 (g)
0.600 moles of SO2 and 0.600
moles of O2 are present in a 4.00 L flask at equilibrium at 100oC.
If the Keq = 680, calculate the SO3 concentration at 100oC.
Keq = [SO3]2
[SO2]2[O2]
680
= [SO3]2
[0.150]2[0.150]
[SO3]2
= (0.150)(0.150)2(680)
[SO3] = 1.51 M
5. Consider the following equilibrium:
2 NO2(g)
⇄ N2O4(g)
2.00 moles of NO2 and1.60
moles of N2O4 are present in a 4.00 L flask at equilibrium
at 20oC. Calculate the Keq at 20oC .
Keq = 1.60
6. 2 SO3(g) ⇄ 2 SO2(g) +
O2(g)
4.00 moles of SO2 and 5.00
moles O2 are present in a 2.00 L container at 100oC and
are at equilibrium. Calculate the equilibrium concentration of SO3 and the number of moles SO3
present if the Keq = 1.47 x 10-3.
[SO3] = 82.5 M 165 moles SO3
7. If at equilibrium [H2]
= 0.200M and [I2] = 0.200M and Keq=55.6 at 250oC, calculate
the equilibrium concentration of HI.
H2 (g) + I2 (g) ⇄ 2HI (g)
[HI] = 1.49 M
8. 1.60 moles CO, 1.60 moles H2O, 4.00 moles CO2,
4.00moles H2 are found in a 8.00L container at 690oC at
equilibrium.
CO (g) + H2O (g) ⇄ CO2 (g) + H2 (g)
Calculate the value of the equilibrium
constant.
Keq = 6.25
Worksheet #9 Equilibrium Calculations
Solve each problem and show all of
your work.
1.
At equilibrium, a 5.0L flask contains:
0.75 mol of PCl5 0.50 mol of H2O 7.50 mol of HCl
5.00 mol of POCl3
Calculate the Keq for the reaction:
PCl5 (s) + H2O (g) ⇄ 2HCl (g) + POCl3 (g)
Keq = 23
2. Keq=
798 for the reaction:
2SO2 (g) + O2
(g) ⇄ 2SO3 (g).
In a particular mixture at equilibrium,
[SO2]= 4.20 M and [SO3]=11.0 M. Calculate the equilibrium
[O2] in this mixture.
[O2] = 8.60 X 10-3 M
3.
Consider the following equilibrium:
2SO2 (g) + O2 (g) ⇄ 2SO3 (g)
When a 0.600 moles of SO2
and 0.600 moles of O2 are placed into a 1.00 litre container and allowed
to reach equilibrium, the equilibrium [SO3]
is to be 0.250M. Calculate the Keq value.
Keq =1.07
4.
Consider the following equilibrium:
2 NO2(g)
⇄ N2O4(g)
If 2.00 moles of NO2 are
placed in a 1.00 L flask and allowed to react. At equilibrium 1.80 moles NO2
are present. Calculate the Keq.
2 NO2(g) ⇄ N2O4(g)
I 2.00
0.00
![]() C -0.20
0.10
C -0.20
0.10
E 1.80 M
0.10 M
Note the loss of one sig fig
Keq = (0.10)
(1.80)2
Keq = 0.031
5.
2
SO2(g) + O2(g) ⇄ 2 SO3(g)
4.00 moles of SO2 and 5.00
moles O2 are placed in a 2.00 L container at 200oC and allowed
to reach equilibrium. If the equilibrium concentration of O2 is 2.00 M, calculate the Keq
Keq = 0.50
6.
If the initial [H2] = 0.200M,
[I2] = 0.200M and Keq = 55.6 at 250oC calculate the equilibrium
concentrations of all molecules.
H2 (g) + I2 (g) ⇄ 2HI (g)
[HI] = 0.315 M
[H2] = [I2] = 0.042 M
7.
1.60 moles CO and1.60 moles H2O
are placed in a 2.00L container at 690 oC (Keq=10.0).
Calculate all equilibrium concentrations.
CO (g) + H2O (g) ⇄ CO2 (g) + H2 (g)
[CO2] = [H2] = 0.608 M [CO] = [H2O] =
0.192 M
8. SO3(g) + NO(g) ⇄ NO2(g) + SO2(g)
Keq = 0.800 at 100oC. If 4.00 moles of each reactant are placed
in a 2.00L container, calculate all equilibrium concentrations at 100oC.
[NO2] = [SO2]
= 0.944 M [SO3]
= [NO] = 1.06 M
9. Consider
the following equilibrium system:
2NO2(g) ⇌ N2O4
Two sets of equilibrium data are listed for
the same temperature.
Container 1 2.00 L
0.12 moles NO2 0.16
moles N2O4 0.060 M NO2
.080 M N2O4
Container 2 5.00
L 0.26 moles NO2 ? moles N2O4 0.052 M NO2
Determine
the number of moles N2O4 in the second container. Get a
Keq from the first container
and use it for the second container.
[NO2]2
= [0.080] = 22.22
[0.060]2
Keq
= [N2O4]
[NO2]2
22.22 = [N2O4]
[0.052]2
[N2O4] =
0.0600 M 5.00 L x 0.0600 moles = 0.30
moles
1 L
Worksheet #10 Equilibrium Calculations
Solve
each problem and show all of your work in your portfolio.
1.
At equilibrium, a 2.0 L flask contains:
0.200
mol of PCl5 0.30 mol
of H2O 0.60 mol of HCl
0.300 mol of POCl3
Calculate
the Keq for the reaction:
PCl5 (g) + H2O (g) ⇄ 2HCl (g) + POCl3 (g)
Keq = 0.90
2. Keq= 798 for the reaction:
2SO2 (g) +
O2 (g) ⇄ 2SO3 (g).
In
a particular mixture at equilibrium, [SO2]= 4.20 M and [SO3]=
11.0M. Calculate the equilibrium [O2] in this mixture.
[O2] = 8.60 X 10-3 M
3.
Consider the following equilibrium: 2SO2 (g) + O2 (g) ⇄ 2SO3 (g)
When
a 0.600 moles of SO2 and 0.600 moles of O2 are placed into
a 2.00 litre container and allowed to reach equilibrium, the equilibrium [SO3]
is to be 0.250M. Calculate the Keq value.
(3
marks)
2SO2
(g) + O2 (g)
⇄ 2SO3
(g)
I
0.300 0.300 0
C
0.250 0.125 0.250
 E
0.050 0.175 0.250
E
0.050 0.175 0.250
Keq
= (0.250)2
(0.050)2(0.175)
Note the loss of one sig fig!
Keq =
4.
H2 (g) + S (s) ⇄ H2S (g)
Keq= 14
0.60
moles of H2 and 1.4 moles of S are placed into a 2.0L flask and allowed
to reach equilibrium. Calculate the [H2] at equilibrium. (4 marks)
Don’t
count S. It is a solid!
[H2]
= 0.02 M
5.
Keq=0.0183 for the reaction:
2HI
(g) ⇄ H2 (g) + I2
(g)
If
3.0 moles of HI are placed in a 5.00L vessel and allowed to reach equilibrium,
what is the equilibrium concentration of H2?
[H2]
= 0.064 M
6.
Consider the equilibrium:
I2
(g) + Cl2 (g) ⇄ 2ICl (g) Keq=
10.0
The
same number of moles of I2 and Cl2 are placed in a 1.0L
flask and allowed to reach equilibrium. If the equilibrium concentration of ICl
is 0.040 M, calculate the initial number of moles of I2 and Cl2.
I2
(g) + Cl2 (g) ⇄ 2ICl (g)
Keq = 10.0
I x
x 0
C 0.020
0.020 0.040
E x – 0.020 x - 0.020 0.040
(0.040)2 =
10.0
(x – 0.020)2
.04 =
3.1622
(x – 0.020)
.04 =
-0.063244 +
3.1622x
0.103244 = 3.1622x
x =
0.033 M
1.0 L
x 0.033 mole = 0.033 mole
L
7.
Consider the equilibrium: 2ICl(g) ⇄ I2 (g) + Cl2 (g)
Keq= 10.0
If x moles of ICl were placed in a 5.0 L container at 10 oC and if an equilibrium concentration of I2 was found to be 0.60 M, calculate the number of moles ICl initially present.
2ICl(g) ⇄ I2 (g) + Cl2
(g) Keq=
10.0
I x
0 0
C 1.2
0.60 0.60
E x – 1.2
0.60 0.60
(0.60)2 = 10.0
(x – 1.2)2
0.60 = 3.162
(x – 1.2)
0.60 = 3.162x
- 3.7944
4.3944
= 3.162x
x =
1.3896 M
5.0 L
x 1.3896 moles = 6.9
moles
L
8.
A student places 2.00 moles SO3 in a 1.00 L flask. At equilibrium [O2]
= 0.10 M at 130 oC. Calculate the Keq.
2SO2(g)
+ O2(g) ⇄ 2SO3(g)
I
0 0 2.0
Note this reaction starts with a product and shifts left to go to equilibrium.
C +.20
+.10
- 0.20
So add on the left and subtract on the right.
E .20
.10 1.8
Keq = (1.8)2 = 810
(0.1)(.2)2
Worksheet #11 Review, Ktrial, & Size
of Keq
1. 2 CrO4-2 (aq)
+ 2H+ (aq) ⇄ Cr2O7-2
(aq) + H2O (l)
Calculate the Keq if the following amounts were found
at equilibrium in a 2.0L volume.
CrO4-2 = .030 mol, H+
= .020 mol, Cr2O7-2 = 0.32 mol, H2O
= 110 mol
Do not count water. It is a liquid!!
Keq =
(0.16)
(0.015)2(0.010)2
Keq = 7.1 X 106
2. PCl5 (s) + H2O (g)
⇄ 2HCl (g) + POCl3 (g) Keq= 11
At equilibrium the 4.0L flask contains the indicated
amounts of the three chemicals.
PCl5 .012
mol H2O .016
mol HCl .120 mol
Calculate [POCl3].
Keq = [HCl]2[POCl3]
[H2O]
11
= [0.030]2[POCl3]
[.0040]
[POCl3]
= (11)(0.0040)
[0.030]2
[POCl3]
= 49
3. 6.0 moles H2S are placed in
a 2.0L container. At equilibrium 5.0 moles H2 are present. Calculate
the Keq
2H2S
(g) ⇄ 2H2
(g) + S2 (g)
I
3.0 0 0
C
2.5 2.5 1.25
![]() E
0.5 2.5 1.25
E
0.5 2.5 1.25
Note the loss of 1 significant digit
Keq = (2.5)2(1.25)
(0.5)2
Keq = 3 x 101
4. 4.0 moles H2 and 2.0 moles
Br2 are placed in a 1.0L container at 180oC. If the [HBr]
= 3.0 M at equilibrium, calculate the Keq.
H2
(g) +
Br2 (g) ⇄ 2HBr (g)
I 4.0
2.0 0
C -1.5
-1.5 +3.0
E 2.5
0.5 3.0
![]()
Keq = (3.0)2 Note the loss
of significant digits here
(2.5)(.5)
Keq = 7
5. At 2000C Keq= 11.6 for 2NO(g) ⇄ N2 (g)
+ O2 (g). If some NO is placed
in a 2.0 L vessel and the equilibrium [N2] = 0.120 M, calculate all
other equilibrium concentrations
2NO(g)
⇄ N2
(g) + O2 (g)
I
x 0 0
C 0.240
0.120 0.120
E x – 0.240 0.120
0.120
(0.120)2
= 11.6
(x – 0.240)2
0.120
= 3.4058
x – 0.240
x = 0.275
M
[N2]
= [O2] = 0.120 M
[NO] = 0.035 M
6.
At 800oC, Keq = 0.279 for CO2 (g) + H2 (g)
⇄ CO (g) + H2O (g).
If 2.00 moles CO( g) and 2.00 moles H2O
(g) are placed in a 500 ml container, calculate all equilibrium concentrations.
Note that when two products are placed
in a container it shifts to the left to get to equilibrium.
CO2 (g)
+ H2 (g)
⇄ CO (g) + H2O (g).
I
0 0 4.00 4.00
C
x x x x
E
x x 4.00 - x 4.00 - x
0.279 = (4-x)2
(x)2
0.5282 = 4
- x
x
0.5282x = 4
– x
1.5282x = 4
[CO2] = [H2] = x = 2.62 M
[CO] = [H2O]
= 4.00 - x = 1.38M
7. CO (g) + H2O (g) ⇄ CO2 (g)
+ H2 (g) Keq=
10.0 at 690oC. If at a
certain time [CO] = 0.80M, [H2O] = 0.050M, [CO2] = 0.50M
and [H2] = 0.40M, is the reaction at equilibrium? If not, how will
it shift in order to get to equilibrium
Ktrial = 5 Keq
= 10 -therefore the reaction is not
at equilibrium and shifts right
8. For the reaction: CO (g) + H2O (g)
⇄ CO2 (g) + H2 (g) Keq= 10.0 at 690oC. The following concentrations were observed:
[CO] =2.0M, [H2] = 1.0M, [CO2]=2.0M, [H2O] =
0.10M. Is the reaction at equilibrium? If not, how will it shift in order to get
to equilibrium?
Ktrial = 10 Keq = 10
- therefore the reaction is at equilibrium
9. For the same equation above the following concentrations were
observed: [CO] = 1.5M, [H2] = 1.2, [CO2] = 1.0M, [H2O]
= .10M. Is the reaction at equilibrium? If not, how will it shift in order to
get to equilibrium?
Ktrial = 8 Keq
= 10 -therefore the reaction is not
at equilibrium and shifts right
10.
At a certain temperature the Keq for
a reaction is 75. 2O3(g) ⇄ 3O2(g)
Predict the direction in which the equilibrium will
proceed, if any, when the following amounts are introduced to a 10 L vessel.
a) 0.60 mole
of O3 and 3.0 mol of O2
Ktrial = (0.30)3 =
7.5 <
Keq Therefore the reaction will shift to the right
to reach equilibrium.
(0.060)2
b) 0.050 mole of O3 and 7.0 mol of O2
Ktrial = (0.70)3
= 13720
> Keq
Therefore the reaction will shift to the left to reach equilibrium.
(0.0050)2
) 1.5 mole of O3 and no O2
Ktrial = (0)3
= 0
< Keq Therefore the reaction will shift to the right to reach equilibrium.
(0.15)2
11.
Consider the following equilibrium:
a) 2NO2 (g)
⇄ N2O4
(g)
Keq = 2.2
b) Cu2+(aq) +
2Ag(s) ⇄ Cu(s) +
2Ag+ (aq)
Keq = 1 x 10-15 Favors reactants to the greatest extent
c) Pb2+ (aq) + 2
Cl- (aq) ⇄ PbCl2(s)
Keq = 6.3 x 104 Favors products to the greatest extent
d) SO2(g) + O2 (g)
⇄ SO3 (g) Keq
= 110
i) Which
equilibrium favors products to the greatest extent?
ii) Which
equilibrium favors reactants to the greatest extent?
12. What is the only way to change the value
of the Keq?
The only way to
change the value of the Keq is by changing the temperature.
13.
In the reaction: A + B ⇄ C + D + 100kJ, what happens
to the value of Keq if we increase the temperature?
The Keq
will decrease.
14.
If the value of Keq decreases when
we decrease the temperature, is the reaction exothermic or endothermic?
The reaction
is endothermic.
15.
In the reaction; W + X + 100kJ ⇄ Y + Z, what happens to the
value of Keq if we increase the [X]? Explain your answer.
The Keq will remain
the same because the only way to change Keq is by changing the temperature.
16.
If the value of Keq increases when
we decrease the temperature, is the reaction exothermic or endothermic?
The reaction
is exothermic.
17.
Predict whether reactants of products
are favored in the following equilibrium systems
(a)
CH3COOH(aq) ⇋ H+(aq) + CH3COO-(aq)
Keq = 1.8 x 10-5 Reactants
(b) H2O2(aq) ⇋ H+(aq) + HO2(aq) Keq
= 2.6 x 10-12 Reactants
(c) CuSO4(aq)
(+ Zn(s) ⇋ Cu(s) + ZnSO4(aq) Keq = 1037
Products
18.) What
effect will each of the following have on the Keq
of the reaction shown below:
2NO2(g) +
heat ⇋ N2O4(g)
Keq = 2.2
(a) adding a catalyst
Remains constant
(b) increasing the concentration of a reactant
Remains constant
(c) increasing the concentration of a product Remains
constant
(d) decreasing the volume
Remains constant
(e) decreasing the pressure
Remains constant
(f) increasing the temperature
Increases
(g) decreasing the temperature
Decreases
Worksheet #12 Enthalpy & Entropy
For
each of these processes, predict if Entropy increases or decreases.
1.
2H2(g) + O2(g) ⇋ 2H2O(g) decreases
2.
2SO3(g) ⇋ 2SO2(g) +
O2(g) increases
3. Ag+(aq) +
Cl-(aq) ⇋ AgCl(s) decreases
4. Cl2(g) ⇋ 2Cl(g) increases
5. H2O(l) ⇋ H2O(g)
increases
6. CaCO3(s) + 180
KJ ⇋ CaO(s) +
CO2(g) increases
7. I2(s) + 608
KJ ⇋ I2(aq)
increases
8. 4Fe(s) + 3O2(g) ⇋ 2Fe2O3(s) + 1570 KJ decreases
Consider
both Enthalpy and Entropy and determine if each reaction will
a)
go to completion
b)
not occur or
c)
go to equilibrium
9.
H2O(l) ⇄ H2O(g) DH = 150 KJ
min enthalpy ⇄ max entropy
Equilibrium
10. CaCO3(s) + 180
KJ ⇄ CaO(s) +
CO2(g)
min enthalpy ⇄ max entropy
Equilibrium
11. I2(s) ⇄ I2(aq) +
608 KJ
⇄ max entropy min enthalpy
Completion
12. 4Fe(s) + 3O2(g) ⇄ 2Fe2O3(s) ΔH = 1570 KJ
Does not Occur
13. Cl2(g) ⇄ 2Cl(g) DH = +26.8 KJ
min enthalpy ⇄ max entropy
Equilibrium
14. Ag+(aq) + Cl-(aq) ⇄ AgCl(s) + 86.2 KJ
min entropy ⇄ min enthalpy
Equilibrium
Considerboth
Enthalpy and Entropy and determine if each reaction will
a)
have a large Keq
b)
have a small Keq
c)
have a Keq about equal to 1
15.H2SO4(aq)
+ Zn(s) ⇋ ZnSO4(aq) +
H2(g) DH +207 KJ
Keq about 1
16.NH4NO3(s)
⇋ NH4+(aq) + NO3-(aq) DH = -30 KJ
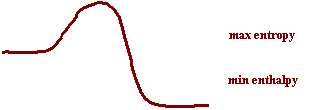
Large Keq
17.N2(g) + 3H2(g)
+ 92 KJ ⇋ 2NH3(g)

Small
Keq
18. H2O(l) + 150 KJ ⇋ H2O(g)
Keq about equal to 1
19.Ca(s) + H2O(l) ⇋ Ca(OH)2(aq) +
H2(g) DH = +210 KJ
Keq about 1