Power Point Lesson Notes- double click on the lesson number.
![]()
Worksheet Answers Quiz Answers
1 Monitoring
Reaction Rates WS 1 Q1
2 Factors that Change the Rate WS 2 Q2
6 Lab: The Iodine Clock Reaction Web Review
7 Review Practice Test 1 Practice Test 2
8. Review Quizmebc
9. Test
Text
book Hebden Read Unit I
The following workbook is designed to ensure that you can demonstrate your understanding of all aspects of the kinetics unit. The minimum expectation is that you do all of these questions by the due dates given by your teacher. Do the questions. Use your notes from class to assist you. Then after you have finished go to the web site to evaluate your work. Make a list of those things that you don’t quite understand and bring them to class. I will go over them as best as I can. There are other things that you should do to prepare for the test at the end of the unit. Remember, what you put into this course is what you will get out. There is no substitute for consistent effort and hard work. If you can’t do a question, get some help before the end of the unit, you need to know, understand, and remember everything. Good luck! I know you can do well in this unit. Web Site Address: iannonechem.com
Ws #1 Monitoring and Calculating Reaction
Rates
1. Read
unit 1 of Hebden over the next week. “A” students should read it twice.
2.
a) When measuring a property associated with a reactant in a reaction, does it
increase or decrease?
Decrease
as reactants are converted into products
2.
b) When measuring a property associated with a product in a reaction, does it
increase or decrease?
Increase
as reactants are converted into products
3.
Give three ways to measure the rate of the following reaction. State the
specific properties that you would monitor and include units (amount is not a
specific property). State if each property would increase or decrease. Describe
in each case how you would calculate the reaction rate.
2HNO3(aq) +
Cu(s) → NO2(g) +H2O(l) + CuNO3(aq)
The first one is done for you.
i) Mass of Cu Grams Decrease Rate = mass/time
ii) [HNO3] M Decrease Rate = M/time
iii) Volume of NO2 L Increase Rate = L/time
iv) [CuNO3] M increase Rate = M/time
v) Mass of open
container Grams Decrease Rate = mass/time
vi) Pressure of
closed container KPa Increase Rate = Pressure/time
Any three of the above answers are fine.
Mass
of copper (g) 3.26 2.93 2.61
Time
(min) 5.0 7.0 9.0
4.
Calculate the rate in units of (g Cu/min).
3.26 - 2.61 g Cu = 0.16g/min
9.0 - 5.0 min
5.
Calculate the rate in units of (mole Cu/min).
0. 16g Cu
x 1 mol = 0.0026
mol/min
min 63.5 g
6.
Calculate the rate in moles HNO3 consumed per second (remember that
2 moles are consumed per 1 mole of Cu).
0.0026 mol Cu x 1 min x 2 moles HNO3 = 8.5
x 10-5 moles/s
min 60s 1 mole Cu
7. Calculate the rate in units of (g/sec) for
HNO3.
8.5 x 10-5 moles HNO3 x 63.0 g = 0.0054 g/s
s 1 mole
Volume of NO2 (mL) 10.0 11.5 12.7
Time (sec) 0.00 5.00 10.00
8. Calculate the rate in units of (mL NO2/sec).
Rate = 12.7 - 10.0 ml
= 0.27 ml/s
10.00
- 0.00 s
9.
Calculate the rate in units of (L NO2/min).
0.27 ml x
60s x 1L
= 0.016 L/min
s
1 min 1000 ml
10.
Calculate the rate in units of (moles NO2/min) at STP.
0.0162 L
x 1 mol = 7.2 x 10-4 mol/min
min
22.4 L
11.
Calculate the rate in units of (moles HNO3/min) at STP (remember
that 2 moles are consumed per 1 mole of NO2)
7.23 x 10-4 mol NO2 x 2
moles HNO3 = 0.0015
moles/min
min 1 mole NO2
12.
Calculate the rate of the following reaction:
2NO (g) +
2H2 (g)
→ N2 (g) +
2H2O (g)
Rate = (0.080
– 0.020) moles = 0.0060 moles/s
(12.0
– 2.0) s
a) What is the rate in moles NO per second? 0.0060 moles/s
b) What is the rate in moles N2 per
second? 0.0030 moles/s
c) What is the rate in grams NO per min? 11 g/min
d) What is the rate in grams N2 per hour? 3.0 x 102g/h
13.
Choose three properties that you could measure in order to monitor the rate of
the following reaction.
Cu (s) +
2AgNO3 (aq)
→ 2 Ag (s) +
Cu(NO3)2 (aq)
Property Unit of Measurement Change
1. Mass Cu grams decrease
2. Mass Ag grams increase
3. Intensity
[Cu+2] M increase
14.
Calculate the rate of the following reaction in units of M/s:
Zn
(s) +
2HCL (aq) → ZnCl2 (aq) +
H2 (g)
Molarity
of HCL (M) 0.612 0.813 1.05
time
(seconds) 21.0 25.0 29.0
Rate = (1.05 - 0.612)
M = 0.055 M/s
(29.0 - 21.0) s
15.
Calculate the rate of the following reaction in L/min:
Zn
(s) +
2HCL (aq) → ZnCl2 (aq) +
H2 (g)
Volume
of H2 (L) 0.255 0.550 0.790
time
(minutes) 1.0 2.0 3.0
Rate = (0.790 -
0.255) M = 0.27 L/min
(3.0 - 1.0) s
16.
If 0.369g of HCl is neutralized with 0.250M NaOH in 25.0 seconds, what is the
reaction rate in moles HCL /min.
0.369g x 1
mole
Rate =
36.5g = 0.0243 mole/min
0.41666 min
WS # 2 Factors
That Change The Reaction Rate
Homogeneous
reactions
- reactants
are in the same phase (aq), (g) , or (l) and are thoroughly mixed.
Heterogeneous
reactions
- reactants
are in the two or more phases and are not thoroughly mixed (two solids do not
mix).
Classify as Homogeneous or
Heterogeneous:
1. Zn (s)
+ 2 HCl (aq) → H2
(g) + ZnCl2 (aq) heterogeneous
2. Ag+
(aq) + Cl- (aq) → AgCl (s) homogeneous
3. H2 (g) + F2 (g) → 2HF (g) homogeneous
4. 2Al (s) + 3I2 (s) → 2AlI3 (s) heterogeneous
The
following four factors will increase the rate of a chemical reaction that is
homogeneous:
1. Increasing the temperature.
2. Increasing the reactant concentration.
3. Adding a catalyst
4. Changing the nature of the reaction.
5. Increasing
the pressure for gases
The above four factors as well as the two below
will increase the rate of a heterogeneous reaction:
6. Increasing the surface area of a solid.
7. Agitation
Which factor
will only increase the rate of a gaseous reaction?
8. Pressure
For each reaction
specifically describe all of the ways to increase the reaction rate
(i.e.. increase[H2]).
1. H2 (g) + F2
(g) → 2 HF (g) This reaction is
homogeneous so the first four factors will work.
Increasing the temperature
Increasing the pressure
Increasing [H2] or [F2]
Adding a catalyst
2. HCl (aq)
+ NaOH (aq) →
NaCl (aq) + H2O (l) This reaction is homogeneous so the first four factors
will work.
Increasing the temperature
Increasing [HCl] or [NaOH]
Adding a catalyst
3. Zn (s) + 2
HCl (aq) → H2 (g) + ZnCl2 (aq) This reaction is heterogeneous most of the factors will
work, except pressure- need a gaseous reactant..
Increasing the temperature
Increasing [HCl]
Adding a catalyst
Increasing the surface area of Zn(s)
Agitation
4. State three examples of chemical reactions that are desired to
be slow.
Food spoiling
Metal corrosion
Erosion
5. Give three examples of chemical reactions that are desired to
be fast.
Combustion of gasoline in
automobiles
Industrial chemical production
Cooking food
The combustion of gasoline in a car
engine; while accelerating.
6. List all of the ways to increase the rate of the following
reaction:
H2O2 (aq) →
H2 (g) + O2 (g)
Increasing the temperature
Increasing [H2O2]
Adding a catalyst
I.
Homogeneous reactions are generally faster than heterogeneous- the reactants are
mixed better and therefore there are more collisions between reactant
particles.
HCl (aq) + NaOH (aq) → NaCl (aq)
+ H2O (l)
is faster than
Zn (s) + 2
HCl (aq) → H2 (g) + ZnCl2 (aq)
II. Simple ionic reactions (where there are no bonds
to break) are generally faster than more complex ionic reactions (where there
are bonds to break).
Pb+2 (aq) +
2Cl- (aq) →
PbCl2 (l)
is faster than
2Na+ (aq) +
2ClO- (aq) → 2Na+ (aq) + 2Cl-(aq) + O2 (g)
1.
Indicate the faster and slower reaction and explain why.
a)
2Al (s) + 3I2 (s) → 2AlI3 (s)
Heterogeneous
reaction with bonds to break will be slow.
b)
Ag+(aq) + Cl-(aq) → AgCl
(s)
Homogeneous
reaction with no bonds to break will be fast.
2.
Indicate the faster and slower reaction and explain why.
a)
2Al (s) + 3I2 (s) → 2AlI3 (s)
Slow.
The reaction is heterogeneous (two solid do not mix) with bonds to break.
b)
2Na+ (aq) + 2ClO- (aq) → 2Na+ (aq) + 2Cl-(aq) + O2 (g)
Faster.
The reaction is homogeneous.
3.
Indicate the faster and slower reaction and explain why.
a)
3Ba+2(aq)
+ 2PO4-3
(aq) → Ba3(PO4)2(aq)
Faster.
The reaction is homogeneous and simple ionic with no bonds to break.
b)
Cu(s) + 2Ag+(aq) →
Cu+2 (aq)
+ 2Ag (s)
Slow. The
reaction is heterogeneous and the Cu(s) bonds need to be broken.
1. Chemical reactions are the result of collisions
between reactant particles, where bonds
are broken and new ones form.
2. A successful collision requires sufficient energy and favorable geometry.
3. Describe as fast, medium or slow. Explain!
i) 2 H2 (g)
+ O2 (g) → 2 H20 (l) (room temperature)
Slow. Gas reactions are
slower than aqueous.
ii) 2 Ag+ (aq)
+ CO32- (aq) → Ag2CO3
(s)
Fast.
Homogeneous reaction simple ionic- there are no bonds to break
iii) 2 HCl
(aq) + Na2CO3 (aq) → CO2
(g) + 2 NaCl (aq) + H20 (l)
Medium. Homogeneous complex reaction
- there are bonds to break.
4. i) Describe how you would measure the rate
of the reaction :
Zn (s) + 2 HCl (aq) →
ZnCl2 (aq) + H2 (g)
Measure
the decrease in Zn mass.
Measure the increase in H2
gas volume.
Measure the mass of an open
container which decreasing due H2 escaping.
ii) List four ways to increase the rate.
Increasing the temperature
Increasing [HCl]
Adding a catalyst
Increasing the surface
area of Zn(s)
Agitation
5. A 10 °C temperature increase frequently doubles the rate of a
slow reaction because:
a) The temperature has doubled.
b) The PE of the colliding particle has doubled.
c) The KE of the colliding particle has doubled.
d) The fraction of
particles with sufficient KE to react has doubled.
6. Both collisions A and B have the same KE. Which collision is
successful and explain why.
Before
Collision After
Collision
![]()
![]()
![]()
A)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() B)
B)
Collision B was successful due
to favourable geometry.
7. Use the collision theory to explain how each factor increases
the reaction rate.
i) Increasing
temperature i) more
collisions and harder collisions
ii) Increasing
[reactants] ii) more collisions
iii) Increasing
surface area (solid) iii) more
collisions
iv) Agitation
of a heterogeneous reaction iv) more
collisions
v) Adding
a catalyst v) lower Ea
& low energy collisions are successful
8. Explain why collision A was successful while collision B was
unsuccessful.
Before
Collision After
Collision
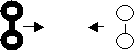
A)
![]()
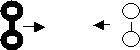

B)
Collision
A was successful because it had sufficient energy. The geometry is the same for
both collisions.
Explain each of the
following using the collision theory. You need to explain each statement.
9.
· a candle is not burning at
room temperature Ea is
too high
· a match lights the candle Provides Ea
· the candle continues to burn Exothermic
10.
· H2O2
decomposes slowly at 20o C Ea is too
high
· KI is added and rapid
decomposition begins Catalyst- lowers Ea
· The temperature increases Exothermic
11.
· H2 and O2
in a balloon do not react Ea is too high
· A spark ignites the balloon Provides Ea
· An explosion results Exothermic
12.
· CH4 and O2
in a balloon do not react Ea is too high
· A platinum gauze ignites the
balloon Catalyst lowers Ea
· An explosion results Exothermic
13.
N2(g) + O2(g) → 2NO(g)
Even
though there are more than four billion collisions per second between N and O
the amount of product after a year is too small to detect. Using the collision
theory, give two reasons why this reaction might be slow.
i) Low
Temperature
ii)
High
Ea
14.
Give two reasons why some collisions will not result in a chemical reaction.
i) Insufficient
energy
ii)
Poor
geometry
15.
Give five reasons that might account for the following reaction having a high
rate.
Ca (s)
+ 2 HCl (aq)
→ CaCl2 (aq) + H2 (g)
i) High
surface area of Ca
ii)
High
concentration of HCl
iii)
High
temperature
iv)
Agitation
v) Nature of
the reactant
16.
C(s) + O2(g) → CO2(g)
List
four ways the rate of the reaction could be increased.
i) Increase
temperature
ii)
Increase
[ O2 ]
iii) Increase
pressure
iv) Increase SA
of C
(add catalyst or agitate)
17.
State the relationship between Activation energy and the rate of a reaction.
Graph the relationship.
Inverse because decreasing the
activation energy increases the rate.
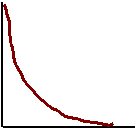
Rate
Activation Energy
18.
State the relationship between Temperature and the rate of a reaction. Graph
the relationship.
Direct, because increasing the
temperature increases the rate.

Rate
Temperature
19.
State the relationship between Concentration and the rate of a reaction. Graph the
relationship.
Direct, because increasing the
concentration increases the rate.

Rate
20. Give three examples of reactions that are desired to be slow.
a) food spoiling
b) corrosion of metal
c) the fading of the
colour in paint
21. Give three examples of reactions that are
desired to be fast.
a) explosions
b) the combustion of gasoline in your car when you are
passing someone on the freeway
c) the commercial production of chemicals
Molarity
22. List all of the ways to increase the rate of the reaction:
2 H2O2 (aq) → 2 H2O
(l) + O2 (g)
Increase the H2O2
concentration
Increase the Temperature
Add KI catalyst
23. Describe how you would measure the rate of the reaction above.
State the property you would measure and describe how it changes. Draw a
diagram to illustrate your answer.
Mass of an open container Decreases
or
Volume of O2 See notes for diagram.
Pressure of O2 in a closed
system. See notes for diagram.
24. Pick the fastest and the slowest reaction at 20 °C.
Slowest gases
are slower than aqueous a) H2(g) + I2(g) → 2 HI(g)
b) 2 HCl(aq) +
Na2CO3(aq) → CO2(g) + 2
NaCl(aq) + H2O(l)
Fastest-
simple ionic or double replacement c) Hg2+(aq)
+ 2 I -(aq) → HgI2(s)
25. H2 and O2 can exist at 20 °C for years
without reacting. But when a small spark ignites the mixture it reacts
explosively. Explain using the Collision Theory.
The activation
energy is too high at room temperature so there are no successful collisions.
A spark provides
the kinetic energy required to overcome the Ea.
Exothermic
reactions produce energy.
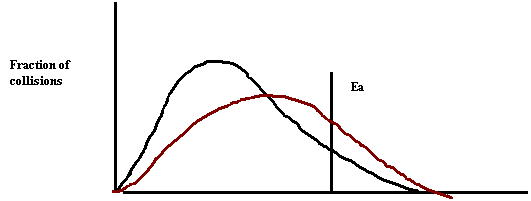
26. Draw a collision energy distribution diagram for a reaction where the y-axis is fraction of collisions and the x-axis is collision energy. Draw the Ea line showing about 10% of the collisions having sufficient energy. Draw the Ea line for the catalyzed reaction where 20% have sufficient energy.
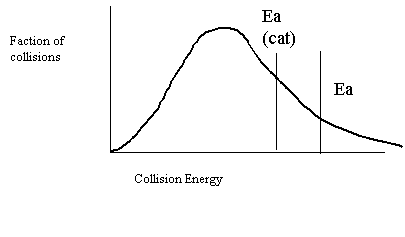
27. Shade in the area of the collision energy distribution diagram showing those collisions that do not have the required energy to be successful at the temperature below.
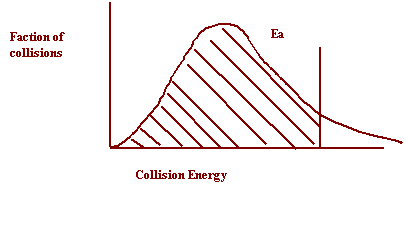
28. Shade in the area of the collision energy distribution diagram showing those collisions that do have the required energy to be successful at the temperature below. Redraw the curve at a higher temperature.

Collision Energy
Kinetics - Descriptions
Use the collision theory to explain the following. Each sentence must
be explained with a statement from the collision theory.
1. An unlit candle does not
burn. It burns after being lit with a match. It continues to burn.
Ea is too high.
Match is energy and provides Ea.
Exothermic
2. A solution is reacting
very slowly to produce bubbles. KI is added and although it is not consumed in
the reaction , it speeds up the reaction rate. The temperature increases. The
rate increases even more.
Ea is high
KI is a catalyst and lowers Ea and more
collision are successful.
Exothermic→ temperature increases
→ rate increases
3. Iron reacts slowly with
HCl. Iron is replaced with Zn and a much more vigorous reaction rate occurs.
Nature of reactant Fe → high Ea Zn → low
Ea
4. H2 and O2
can exist together for years at room temperature without reacting. A spark
begins the reaction. An explosion results.
High Ea
→ collisions are not successful
A spark provides the Ea
Exothermic → explosion
5.
Dilute nitric acid shows little reaction with copper. Concentrated nitric acid
vigorously reacts.
Low concentration → few collisions
High concentration → many collisions
6.
Water puts out a fire.
Lowers temperature so there are less
collisions
The collisions have less energy.
7. Paint prevents rusting.
There are fewer collisions between
reactant molecules.
8. A preservative in food
slows rotting.
The preservative is an inhibitor; which
increases the Ea.
Ws # 4 Potential Energy Diagrams Worksheet
1. Draw the PE diagram showing the PE changes that occur during a
successful collision of the exothermic reaction:
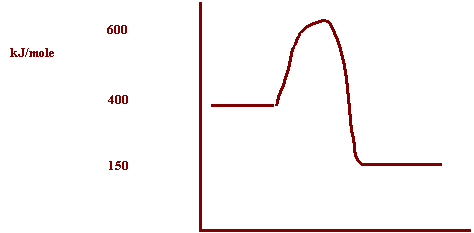
H2 + I2 → 2 HI + 250 KJ
The PE of the reactants = 400 KJ
The activation energy of the forward reaction = 200 KJ
Reaction path
2. Draw the PE diagram showing the PE changes that occur during a
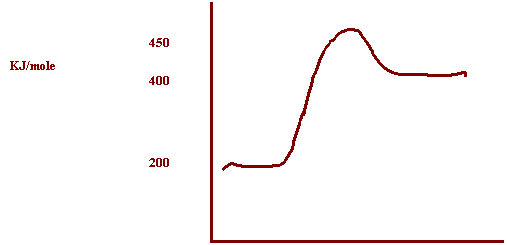
successful collision of the endothermic reaction:
A
+ B + 200 KJ → C
The PE of the reactants = 200 KJ
The Activation Energy in the forward direction = 250 KJ
Reaction Path
3.
Write the following reaction in ΔH notation.
A
+ B + 200 kJ → C
A + B
-----> C ΔH= +200kJ
4.
Write the following reaction in Standard Notation.
H2 +
I2 → 2 HI
ΔH = -250 kJ
H2 +
I2 →
2HI + 250 kJ
5.
Write in Standard Notation.
2NI3 +
3BaCl2 → 2NCl3 + 3BaI2 ΔH = 175 kJ
2NI3 +
3BaCl2 + 175 kJ → 2NCl3 + 3BaI2
6.
Write in ΔH notation.
2AlBr3 +
3BaF2
→ 2AlF3 +
3BaBr2 + 276 kJ
2AlBr3 +
3BaF2 → 2AlF3 + 3BaBr2 ΔH= -267 kJ
Draw
the potential energy diagram for the following reactions.
7. Potential energy
of reactants = 250 kJ
Potential
Energy of activated complex = 350
kJ
Potential
Energy of the products = 300
kJ
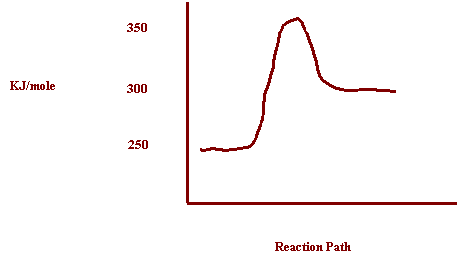
a)
How does the potential energy change as the reaction proceeds? Increases
b)
How does the kinetic energy change as the reaction proceeds? Decreases
c)
Is the reaction exothermic or endothermic? Endothermic
d)
What is the value of ΔH? ΔH= +50kJ
If
a catalyst was added, what would happen to the energies of the:
e)
Reactants? Nothing
f)
Products? Nothing
g)
Activated Complex? Decrease
h) If
a catalyst was added what would happen to the rate? Increase
Draw
the potential energy diagram for the following reactions.
8. Potential energy
of reactants = 350 kJ
Activation
Energy = 100
kJ
Potential
Energy of the products = 250
kJ
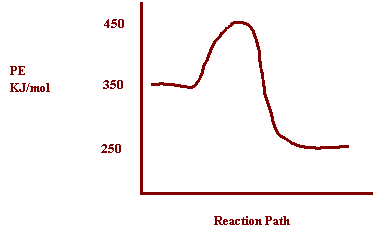
a)
How does the potential energy change as the reaction proceeds? Decreases
b)
How does the kinetic energy change as the reaction proceeds? Increases
c) Is
the reaction exothermic or endothermic? Exothermic
d)
What is the value of ΔH? ΔH= -100kJ
If
the concentration of the reactants was increased, what would happen to the
energies of the:
e)
Reactants? Nothing
f)
Products? Nothing
g)
Activated Complex? Nothing
h)
What would happen to the rate? Increase
Draw
the potential energy diagram for the following reactions.
9. Potential energy
of reactants = 200 kJ
Potential
Energy of activated complex = 400
kJ
ΔH = 150
kJ
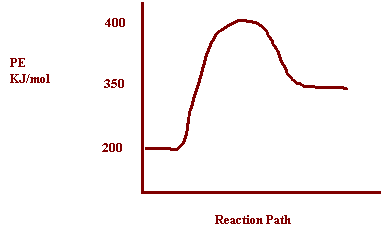
a) How does the potential energy change as the
reaction proceeds? Increases
b) How does the kinetic energy change as the
reaction proceeds? Decreases
c) Is the reaction exothermic or endothermic? Endothermic
d) What is the value of ΔH? ΔH= 150 kJ
If
the temperature was increased, what would happen to the energies of the:
e) Reactants? Nothing
f) Products? Nothing
g) Activated Complex? Nothing
h) What would happen to the rate? Increase
10.
Potential
energy of products = 50 kJ
Potential
Energy of activated complex = 400
kJ
ΔH= -50
kJ
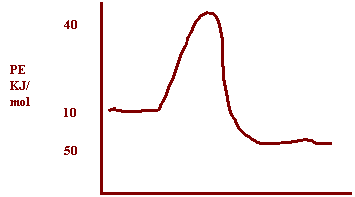
Reaction Path
a)
How does the potential energy change as the reaction proceeds? Decreases
b)
How does the kinetic energy change as the reaction proceeds? Increases
c)
Is the reaction exothermic or endothermic? Exothermic
d)
What is the value of ΔH? ΔH= -50kJ
If
the surface area of the reactants was increased, what would happen to the
energies of the:
e)
Reactants? Nothing
f)
Products? Nothing
g)
Activated Complex? Nothing
h)
What would happen to the rate? Increase
11.
What is the only thing, other than changing the reaction that will change the
potential energy diagram? Describe how it will effect the diagram and the rate.
Catalyst Lowers Ea alloys more low energy
collisions to be successful and increase the rate.
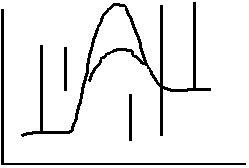
12. Label each
interval on the potential energy diagram. a
b c d e
a) Ea (forward)
(catalyzed)
PE
b) Ea
(reverse)(catalyzed)
c) ΔH
d) Ea
(forward) (uncatalyzed)
Reaction Path
e) Ea
(reverse) (uncatalyzed)
12. Label each interval on the potential energy
diagram.
a b c d e
a) Ea
(forward) (uncatalyzed)
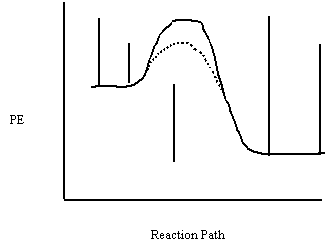
b) Ea
(forward) (catalyzed)
c) ΔH
d) Ea
(reverse) (uncatalyzed)
e) Ea
(reverse) (catalyzed)
1. OCl- +
H2O → HOCl + OH-
HOCl +
I- →
HOI + Cl-
HOI +
OH- →
H2O + OI-
i) The net chemical equation is: OCl- +
I- + →
Cl- +OI-
ii) The
reaction intermediates are: HOCl HOI OH-
iii) The
catalyst is: H2O
2. Br2 → 2Br fast
Br +
OCl2 → BrOCl + Cl slow
Br +
Cl →
BrCl fast
i) The net chemical equation is: Br2 +
OCl2 → BrOCl
+ BrCl
ii) The
reaction intermediates are: Cl & Br
iii) The
catalyst is: None
iv) The
rate determining step is 2
v) If
the concentration of Br2 is increased will the rate of the reaction
increase? Explain your answer.
No because it
is not in the rate determining step.
vi) If the concentration of OCl2 is
increased will the rate of the reaction increase? Explain your answer.
Yes because,
OCl2 is in the rate determining step.
3.
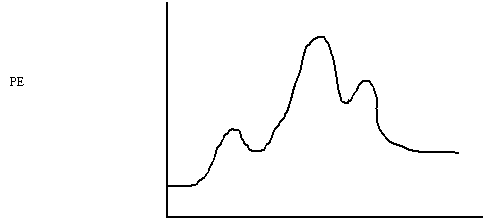
The mechanism for the catalytic decomposition of formic acid is shown below.
step
1 HCOOH +
H+ → [HCOOHH]+
step
2 [HCOOHH]+ → [HCO]+
+ HOH
step
3 [HCO]+ → CO + H+
The potential energy diagram is:
190
180
170
160
150
Reaction Path
i) The catalyst is H+ Crosses
out from left to right
ii) The rate determining step is Two Highest
Ea
iii) ΔH
= +10 kJ From
start to end
iiv) The forward activation energy is 40 kJ Reactants
to the highest point
iv) The reverse activation energy is 30 kJ Products
to the highest point
v) The enthalpy of [HCOOHH]+ is 160 kJ After
one hump
vi) Is the reaction exothermic or endothermic? Endo Uphill
vii) Which chemical formula has the greatest
potential energy? (HCO)+
+ HOH Highest point
on graph
viii) Which chemical formula has the greatest
kinetic energy? HCOOH + H+ Lowest
point on graph
ix) Does this reaction absorb or release kinetic
energy? Absorb because it is endothermic (uphill)
4. Define and remember the following definitions.
mechanism A sequence of steps that determines the overall reaction.
activation energy The minimum energy required in a successful collision.
rate determining step The slowest step in a reaction
mechanism.
catalyst A substance that increases the rate of a chemical reaction
by providing a alternate mechanism with lower activation energy. reaction
intermediate A
chemical species produced in a reaction mechanism and then consumed in a later
step.
endothermic A reaction that absorbs energy
exothermic A reaction that produces energy
activated complex A unstable reaction intermediate with high potential
energy and low kinetic energy.
ΔH The change in enthalpy or heat content for a reaction.
reaction rate The change in a reactant or product per unit of time.
5. The catalyzed decomposition of acetaldehyde has
an overall reaction of:
CH3CHO
→ CH4 +
CO . Determine step 2 of the reaction
mechanism.
A proposed mechanism is:
step 1 CH3CHO +
I2 → CH3I + HI +
CO
step 2 HI +
CH3I → I2 + CH4 This is
the only step 2 that will give the overall reaction below.
overall CH3CHO →
CH4 + CO
6. The following reaction has an overall reaction
of:
2Ce4+ + Tl+ → 2Ce3+
+ Tl3+
Determine step 2 of the reaction mechanism.
A proposed mechanism is:
step 1 Ce4+ +
Mn+2 → Ce3+ + Mn3+
step 2 Ce4+ +
Mn3+ → Ce3+ + Mn4+ This
is the only step 2 that will give the overall reaction below
step 3 Mn4+ +
Tl+
→ Tl3+ + Mn2+
overall 2Ce4+ +
Tl+ → 2Ce3+ + Tl3+
7. A reaction has a overall equation of: Br2 +
OCl2 → BrOCl
+ BrCl . Determine step 3 of
the mechanism.
step 1 Br2 →
2Br
step
2 Br +
OCl2 → BrOCl
+ Cl
step 3 Br + Cl →
BrCl This is
the only step 3 that will give the overall reaction below
overall Br2 +
OCl2 → BrOCl
+ BrCl
List two intermediates: Br Cl
8. Complete the following mechanism.
step 1 NO + Pt →
NOPt needed for next step
step 2 NOPt + NO →
O2Pt + N2 O2Pt needed for next step and N2
needed to be a product
step 3 O2Pt →
O2 + Pt
overall 2NO → N2
+ O2
Identify the catalyst Pt Crosses
out from left to right
Identify the two intermediates NOPt O2Pt Crosses out from right to left
9. Draw a collision energy distribution diagram for a reaction where the y-axis is fraction of collisions and the x axis is collision energy. Draw the Ea line showing about 10% of the collisions having sufficient energy. Draw the Ea line for the catalyzed reaction where 20% have sufficient energy.
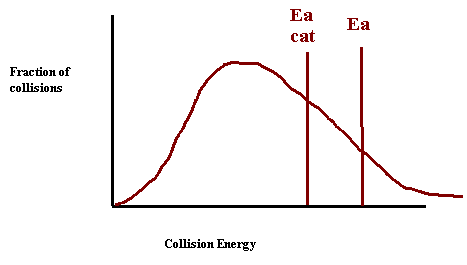
10. Shade in the area of the collision energy distribution diagram showing those collisions that have the required energy to be successful at the low temperature shown below. Draw the curve that represents the distribution at a higher temperature with a different color. Shade in the area representing the successful collisions at the higher temperature with a new color.